The sonic hedgehog signaling pathway stimulates anaplastic thyroid cancer cell motility and invasiveness by activating Akt and c-Met
- PMID: 26859575
- PMCID: PMC4891133
- DOI: 10.18632/oncotarget.7228
The sonic hedgehog signaling pathway stimulates anaplastic thyroid cancer cell motility and invasiveness by activating Akt and c-Met
Abstract
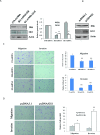
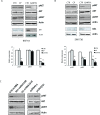
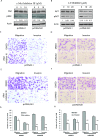
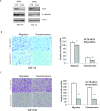
The sonic hedgehog (Shh) pathway is highly activated in thyroid neoplasms and promotes thyroid cancer stem-like cell phenotype, but whether the Shh pathway regulates thyroid tumor cell motility and invasiveness remains unknown. Here, we report that the motility and invasiveness of two anaplastic thyroid tumor cell lines, KAT-18 and SW1736, were inhibited by two inhibitors of the Shh pathway (cyclopamine and GANT61). Consistently, the cell motility and invasiveness was decreased by Shh and Gli1 knockdown, and was increased by Gli1 overexpression in KAT-18 cells. Mechanistic studies revealed that Akt and c-Met phosphorylation was decreased by a Gli1 inhibitor and by Shh and Gli1 knockdown, but was increased by Gli1 overexpression. LY294002, a PI-3 kinase inhibitor, and a c-Met inhibitor inhibited the motility and invasiveness of Gli1-transfected KAT-18 cells more effectively than the vector-transfected cells. Knockdown of Snail, a transcription factor regulated by the Shh pathway, led to decreased cell motility and invasiveness in KAT-18 and SW1736 cells. However, key epithelial-to-mesenchymal transition (EMT) markers including E-cadherin and vimentin as well as Slug were not affected by cyclopamine and GANT61 in either SW1736 or WRO82, a well differentiated follicular thyroid carcinoma cell line. Our data suggest that the Shh pathway-stimulated thyroid tumor cell motility and invasiveness is largely mediated by AKT and c-Met activation with little involvement of EMT.
Keywords: PI-3 kinase; Snail; c-Met; cell motility and invasion; sonic hedgehog signaling pathway.
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Figures


Similar articles
-
The sonic hedgehog signaling pathway maintains the cancer stem cell self-renewal of anaplastic thyroid cancer by inducing snail expression.J Clin Endocrinol Metab. 2014 Nov;99(11):E2178-87. doi: 10.1210/jc.2014-1844. Epub 2014 Jul 31. J Clin Endocrinol Metab. 2014. PMID: 25078145 Free PMC article.
-
Folate deprivation enhances invasiveness of human colon cancer cells mediated by activation of sonic hedgehog signaling through promoter hypomethylation and cross action with transcription nuclear factor-kappa B pathway.Carcinogenesis. 2012 Jun;33(6):1158-68. doi: 10.1093/carcin/bgs138. Epub 2012 Mar 29. Carcinogenesis. 2012. PMID: 22461522
-
Activation of the Sonic Hedgehog pathway in thyroid neoplasms and its potential role in tumor cell proliferation.Endocr Relat Cancer. 2012 Apr 10;19(2):167-79. doi: 10.1530/ERC-11-0305. Print 2012 Apr. Endocr Relat Cancer. 2012. PMID: 22241722
-
Hedgehog target genes: mechanisms of carcinogenesis induced by aberrant hedgehog signaling activation.Curr Mol Med. 2009 Sep;9(7):873-86. doi: 10.2174/156652409789105570. Curr Mol Med. 2009. PMID: 19860666 Review.
-
[Hedgehog signaling in the pathogenesis of neuro-oncology diseases].Biomed Khim. 2015 May-Jun;61(3):332-42. doi: 10.18097/PBMC20156103332. Biomed Khim. 2015. PMID: 26215410 Review. Russian.
Cited by
-
Theory and application of TTFields in newly diagnosed glioblastoma.CNS Neurosci Ther. 2024 Mar;30(3):e14563. doi: 10.1111/cns.14563. CNS Neurosci Ther. 2024. PMID: 38481068 Free PMC article. Review.
-
Targeting GLI1 Transcription Factor for Restoring Iodine Avidity with Redifferentiation in Radioactive-Iodine Refractory Thyroid Cancers.Cancers (Basel). 2022 Mar 31;14(7):1782. doi: 10.3390/cancers14071782. Cancers (Basel). 2022. PMID: 35406554 Free PMC article.
-
GANT61 suppresses cell survival, invasion and epithelial-mesenchymal transition through inactivating AKT/mTOR and JAK/STAT3 pathways in anaplastic thyroid carcinoma.Cancer Biol Ther. 2022 Dec 31;23(1):369-377. doi: 10.1080/15384047.2022.2051158. Cancer Biol Ther. 2022. PMID: 35491899 Free PMC article.
-
An 8 miRNA-Based Risk Score System for Predicting the Prognosis of Patients With Papillary Thyroid Cancer.Technol Cancer Res Treat. 2020 Jan-Dec;19:1533033820965594. doi: 10.1177/1533033820965594. Technol Cancer Res Treat. 2020. PMID: 33054579 Free PMC article.
-
Hedgehog signaling in tissue homeostasis, cancers, and targeted therapies.Signal Transduct Target Ther. 2023 Aug 18;8(1):315. doi: 10.1038/s41392-023-01559-5. Signal Transduct Target Ther. 2023. PMID: 37596267 Free PMC article. Review.
References
-
- Hooper JE, Scott MP. Communicating with Hedgehogs. Nat Rev Mol Cell Biol. 2005;6:306–17. - PubMed
-
- Taipale J, Beachy PA. The Hedgehog and Wnt signalling pathways in cancer. Nature. 2001;411:349–54. - PubMed
-
- van den Brink GR. Hedgehog signaling in development and homeostasis of the gastrointestinal tract. Physiol Rev. 2007;87:1343–75. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

