Interventions for dysphagia in long-term, progressive muscle disease
- PMID: 26859621
- PMCID: PMC8759487
- DOI: 10.1002/14651858.CD004303.pub4
Interventions for dysphagia in long-term, progressive muscle disease
Abstract
Background: Normal swallowing function is divided into oral, pharyngeal, and oesophageal phases. The anatomy and physiology of the oral cavity facilitates an oral preparatory phase of swallowing, in which food and liquid are pushed towards the pharynx by the tongue. During pharyngeal and oesophageal phases of swallowing, food and liquid are moved from the pharynx to the stomach via the oesophagus. Our understanding of swallowing function in health and disease has informed our understanding of how muscle weakness can disrupt swallowing in people with muscle disease. As a common complication of long-term, progressive muscle disease, there is a clear need to evaluate the current interventions for managing swallowing difficulties (dysphagia). This is an update of a review first published in 2004.
Objectives: To assess the effects of interventions for dysphagia in people with long-term, progressive muscle disease.
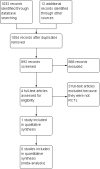
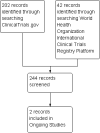
Search methods: On 11 January 2016, we searched the Cochrane Neuromuscular Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, AMED, LILACS, and CINAHL. We checked references in the identified trials for additional randomised and quasi-randomised controlled trials. We also searched ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform on 12 January 2016 for ongoing or completed but unpublished clinical trials.
Selection criteria: We included randomised and quasi-randomised controlled trials that assessed the effect of interventions for managing dysphagia in adults and children with long-term, progressive muscle disease, compared to other interventions, placebo, no intervention, or standard care. Quasi-randomised controlled trials are trials that used a quasi-random method of allocation, such as date of birth, alternation, or case record number. Review authors previously excluded trials involving people with muscle conditions of a known inflammatory or toxic aetiology. In this review update, we decided to include trials of people with sporadic inclusion body myositis (IBM) on the basis that it presents as a long-term, progressive muscle disease with uncertain degenerative and inflammatory aetiology and is typically refractory to treatment.
Data collection and analysis: We applied standard Cochrane methodological procedures.
Main results: There were no randomised controlled trials (RCTs) that reported results in terms of the review's primary outcome of interest, weight gain or maintenance. However, we identified one RCT that assessed the effect of intravenous immunoglobulin on swallowing function in people with IBM. The trial authors did not specify the number of study participants who had dysphagia. There was also incomplete reporting of findings from videofluoroscopic investigations, which was one of the review's secondary outcome measures. The study did report reductions in the time taken to swallow, as measured using ultrasound. No serious adverse events occurred during the study, although data for the follow-up period were lacking. It was also unclear whether the non-serious adverse events reported occurred in the treatment group or the placebo group. We assessed this study as having a high risk of bias and uncertain confidence intervals for the review outcomes, which limited the overall quality of the evidence. Using GRADE criteria, we downgraded the quality of the evidence from this RCT to 'low' for efficacy in treating dysphagia, due to limitations in study design and implementation, and indirectness in terms of the population and outcome measures. Similarly, we assessed the quality of the evidence for adverse events as 'low'. From our search for RCTs, we identified two other non-randomised studies, which reported the effects of long-term intravenous immunoglobulin therapy in adults with IBM and lip-strengthening exercises in children with myotonic dystrophy type 1. Headaches affected two participants treated with long-term intravenous immunoglobulin therapy, who received a tailored dose reduction; there were no adverse events associated with lip-strengthening exercises. Both non-randomised studies identified improved outcomes for some participants following the intervention, but neither study specified the number of participants with dysphagia or demonstrated any group-level treatment effect for swallowing function using the outcomes prespecified in this review.
Authors' conclusions: There is insufficient and low-quality RCT evidence to determine the effect of interventions for dysphagia in long-term, progressive muscle disease. Clinically relevant effects of intravenous immunoglobulin for dysphagia in inclusion body myositis can neither be confirmed or excluded using the evidence presented in this review. Standardised, validated, and reliable outcome measures are needed to assess dysphagia and any possible treatment effect. Clinically meaningful outcomes for dysphagia may require a shift in focus from measures of impairment to disability associated with oral feeding difficulties.
Conflict of interest statement
KJ: Her research contribution to this review has been paid for by grants from Myositis UK and the Association Française contre les Myopathies. KJ was Assistant Managing Editor of Cochrane Neuromuscular at the time of publication. Her work on the review largely predated this appointment.
RP: None known.
MR: None known.
SM: None known.
MH: None known for current review update. She works primarily as a consultant neurologist and Associate Professor at Swansea University. She also has an established private medical practice, and as part of this work could review patients with dysphagia secondary to muscle disease.
UB: None known for current review update. His institution received a consulting fee for trial design and a fee for an ongoing clinical trial of bimagrumab in inclusion body myositis from Novartis.
TH: None known.
Figures
Update of
-
WITHDRAWN: Treatment for swallowing difficulties (dysphagia) in chronic muscle disease.Cochrane Database Syst Rev. 2014 Aug 8;(8):CD004303. doi: 10.1002/14651858.CD004303.pub3. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2016 Feb 09;2:CD004303. doi: 10.1002/14651858.CD004303.pub4. PMID: 25105911 Updated. No abstract available.
Similar articles
-
Treatments for chronic inflammatory demyelinating polyradiculoneuropathy (CIDP): an overview of systematic reviews.Cochrane Database Syst Rev. 2017 Jan 13;1(1):CD010369. doi: 10.1002/14651858.CD010369.pub2. Cochrane Database Syst Rev. 2017. PMID: 28084646 Free PMC article.
-
Interventions for infantile haemangiomas of the skin.Cochrane Database Syst Rev. 2018 Apr 18;4(4):CD006545. doi: 10.1002/14651858.CD006545.pub3. Cochrane Database Syst Rev. 2018. PMID: 29667726 Free PMC article.
-
Modifying the consistency of food and fluids for swallowing difficulties in dementia.Cochrane Database Syst Rev. 2018 Sep 24;9(9):CD011077. doi: 10.1002/14651858.CD011077.pub2. Cochrane Database Syst Rev. 2018. PMID: 30251253 Free PMC article.
-
Corticosteroids for the treatment of Duchenne muscular dystrophy.Cochrane Database Syst Rev. 2016 May 5;2016(5):CD003725. doi: 10.1002/14651858.CD003725.pub4. Cochrane Database Syst Rev. 2016. PMID: 27149418 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
Cited by
-
Silent dysphagia in two patients with Steinert disease and recurrent respiratory exacerbations.Acta Myol. 2020 Sep 1;39(3):141-143. doi: 10.36185/2532-1900-019. eCollection 2020 Sep. Acta Myol. 2020. PMID: 33305171 Free PMC article.
-
Research priorities to improve the health of children and adults with dysphagia: a National Institute of Health Research and Royal College of Speech and Language Therapists research priority setting partnership.BMJ Open. 2022 Jan 25;12(1):e049459. doi: 10.1136/bmjopen-2021-049459. BMJ Open. 2022. PMID: 35078835 Free PMC article.
-
Screening and evaluation tools of dysphagia in adults with neuromuscular diseases: a systematic review.Ther Adv Chronic Dis. 2019 Jan 31;10:2040622318821622. doi: 10.1177/2040622318821622. eCollection 2019. Ther Adv Chronic Dis. 2019. PMID: 30728931 Free PMC article. Review.
-
Role of defective calcium regulation in cardiorespiratory dysfunction in Huntington's disease.JCI Insight. 2020 Oct 2;5(19):e140614. doi: 10.1172/jci.insight.140614. JCI Insight. 2020. PMID: 32897880 Free PMC article.
-
Living with Dysphagia: A Survey Exploring the Experiences of Adults Living with Neuromuscular Disease and their Caregivers in the United Kingdom.J Neuromuscul Dis. 2024;11(2):389-410. doi: 10.3233/JND-230002. J Neuromuscul Dis. 2024. PMID: 38250781 Free PMC article.
References
References to studies included in this review
Dalakas 1997 {published data only}
-
- Dalakas MC, Sonies B, Dambrosia J, Sekul E, Cupler E, Sivakumar K. Treatment of inclusion‐body myositis with IVIg: a double‐blind, placebo‐controlled study. Neurology 1997;48(3):712‐6. [PUBMED: 9065553] - PubMed
References to studies excluded from this review
Dobloug 2012 {published data only}
-
- Dobloug C, Walle‐Hansen R, Gran JT, Molberg Ø. Long‐term follow‐up of sporadic inclusion body myositis treated with intravenous immunoglobulin: a retrospective study of 16 patients. Clinical and Experimental Rheumatology 2012;30(6):838‐42. [PUBMED: 22935197] - PubMed
Horowitz 1987 {published data only}
-
- Horowitz M, Maddox A, Maddern GJ, Wishart J, Collins PJ, Shearman DJ. Gastric and esophageal emptying in dystrophia myotonica. Effect of metoclopramide. Gastroenterology 1987;92(3):570‐7. [PUBMED: 3817383] - PubMed
NCT00773227 {published data only}
-
- NCT00773227. Treatment of dysphagia in oculopharyngeal muscular dystrophy by autologous transplantation of myoblasts. http://clinicaltrials.gov/show/NCT00773227 (accessed 12 January 2016).
Sjögreen 2010 {published data only}
-
- Sjögreen L, Tulinius M, Kiliaridis S, Lohmander A. The effect of lip strengthening exercises in children and adolescents with myotonic dystrophy type 1. International Journal of Pediatric Otorhinolaryngology 2010;74(10):1126‐34. [PUBMED: 20638139] - PubMed
References to ongoing studies
ISRCTN84905610 {published data only}
-
- ISRCTN84905610. Comparative study on treatment methods in cricopharyngeal dysfunction. http://apps.who.int/trialsearch/trial2.aspx?trialid=isrctn84905610 (accessed 12 January 2016).
NCT02328482 {published data only}
-
- NCT02328482. Continuation protocol to protocol BBCO‐001. http://clinicaltrials.gov/show/NCT02328482 (accessed 12 January 2016).
Additional references
Abu‐Baker 2007
-
- Abu‐Baker A, Rouleau GA. Oculopharyngeal muscular dystrophy: Recent advances in the understanding of the molecular pathogenic mechanisms and treatment strategies. Biochimica et Biophysica Acta 2007;1772:173‐85. - PubMed
Archer 2013
-
- Archer SK, Garrod R, Hart N, Miller S. Dysphagia in Duchenne muscular dystrophy assessed by validated questionnaire. International Journal of Language & Communication Disorders 2013;48(2):240‐6. - PubMed
Arvedson 2010
-
- Arvedson J, Clark H, Lazarus C, Schooling T, Frymark T. The effects of oral‐motor exercises on swallowing in children: an evidence‐based systematic review. Developmental Medicine and Child Neurology 2010;52(11):1000‐13. - PubMed
Bülow 2001
-
- Bülow M, Olsson R, Ekberg O. Videomanometric analysis of supraglottic swallow, effortful swallow, and chin tuck in patients with pharyngeal dysfunction. Dysphagia 2001;16(3):190‐5. - PubMed
Cox 2009
Cox 2011
-
- Cox FM, Titulaer MJ, Sont JK, Wintzen AR, Verschuuren JJ, Badrising UA. A 12‐year follow‐up in sporadic inclusion body myositis: an end stage with major disabilities. Brain 2011;134(Pt 11):3167‐75. - PubMed
Darrow 1992
-
- Darrow DH, Hoffman HT, Barnes GJ, Wiley CA. Management of dysphagia in inclusion body myositis. Archives of Otolaryngology ‐ Head and Neck Surgery 1992;118(3):313‐7. - PubMed
Drake 1997
-
- Drake W, O'Donoghue S, Bartram C, Lindsay J, Greenwood R. Eating in side‐lying facilitates rehabilitation in neurogenic dysphagia. Brain Injury 1997;11(2):137‐42. - PubMed
Durmus 2011
-
- Durmus H, Laval SH, Deymeer F, Parman Y, Kiyan E, Gokyigiti M, et al. Oculopharyngodistal myopathy is a distinct entity: clinical and genetic features of 47 patients. Neurology 2011;76(3):227‐35. - PubMed
Ertekin 2001
-
- Ertekin C, Keskin A, Kiylioglu N, Kirazli Y, On AY, Tarlaci S, et al. The effect of head and neck positions on oropharyngeal swallowing: a clinical and electrophysiologic study. Archives of Physical Medicine and Rehabilitation 2001;82(9):1255‐60. - PubMed
Ganger 1990
-
- Ganger D, Craig RM. Swallowing disorders and nutritional support. Dysphagia 1990;4(4):213‐9. - PubMed
Griggs 1995
-
- Griggs RC, Askanas V, DiMauro S, Engel A, Karpati G, Mendell JR, et al. Inclusion body myositis and myopathies. Annals of Neurology 1995;38(5):705‐13. - PubMed
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hill 2002b
-
- Hill M, Hughes T. Workshop: Management of adults and children with feeding difficulties secondary to chronic muscle disease, 22nd March 2002, Sheffield, UK. Neuromuscular Disorders 2002;12(10):970–4. - PubMed
Hughes 1996
-
- Hughes TA, Wiles CM. Clinical measurement of swallowing in health and in neurogenic dysphagia. Quarterly Journal of Medicine 1996;89(2):109‐16. - PubMed
Jaradeh 2006
-
- Jaradeh S. Muscle disorders affecting oral and pharyngeal swallowing. GI Motility Online 2006. [DOI: 10.1038/gimo35] - DOI
Logemann 1994
-
- Logemann JA, Rademaker AW, Pauloski BR, Kahrilas PJ. Effects of postural change on aspiration in head and neck surgical patients. Otolaryngology ‐ Head and Neck Surgery 1994;110(2):222‐7. - PubMed
Low 2001
-
- Low J, Wyles C, Wilkinson T, Sainsbury R. The effect of compliance on clinical outcomes for patients with dysphagia on videofluoroscopy. Dysphagia 2001;16(2):123‐7. - PubMed
Malandraki 2012
-
- Malandraki GA, Kaufman A, Hind J, Ennis S, Gangnon R, Waclawik A, et al. The effects of lingual intervention in a patient with inclusion body myositis and Sjögren's syndrome: a longitudinal case study. Archives of Physical Medicine and Rehabilitation 2012;93(8):1469‐75. - PubMed
Martin 1991
-
- Martin AW. Dietary management of swallowing disorders. Dysphagia 1991;6(3):129‐34. - PubMed
McCann 2007
-
- McCann LJ, Garay SM, Ryan MM, Harris R, Riley P, Pilkington CA. Oropharyngeal dysphagia in juvenile dermatomyositis (JDM): an evaluation of videofluoroscopy swallow study (VFSS) changes in relation to clinical symptoms and objective muscle scores. Rheumatology 2007;46(8):1363‐6. - PubMed
McCullough 2012
Norton 1996
O'Gara 1990
-
- O'Gara JA. Dietary adjustments and nutritional therapy during treatment for oral‐pharyngeal dysphagia. Dysphagia 1990;4(4):209‐12. - PubMed
Oh 2008
-
- Oh TH, Brumfield KA, Hoskin TL, Kasperbauer JL, Basford JR. Dysphagia in inclusion body myositis: clinical features, management, and clinical outcome. American Journal of Physical Medicine and Rehabilitation 2008;87(11):883‐9. - PubMed
Palmer 2010
-
- Palmer PM, Neel AT, Sprouls G, Morrison L. Swallow characteristics in patients with oculopharyngeal muscular dystrophy. Journal of Speech, Language & Hearing Research 2010;53(6):1567‐78. - PubMed
Pandolfino 2005
-
- Pandolfino JE, Kahrilas PJ, American Gastroenterological Association. AGA technical review on the clinical use of esophageal manometry. Gastroenterology 2005;128(1):209‐24. - PubMed
Philpot 1999
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rose 2015
Scott 1998
-
- Scott A, Perry A, Bench J. A study of interrater reliability when using videofluoroscopy as an assessment of swallowing. Dysphagia 1998;13(4):223‐7. - PubMed
Seguy 2002
-
- Seguy D, Michaud L, Guimber D, Cuisset JM, Devos P, Turck D, et al. Efficacy and tolerance of gastrostomy feeding in pediatric forms of neuromuscular diseases. Journal of Parenteral and Enteral Nutrition 2002;26(5):298‐304. - PubMed
Straathof 2014
-
- Straathof CS, Doorenweerd N, Wokke BH, Dumas EM, Bergen JC, Buchem MA, et al. Temporalis muscle hypertrophy and reduced skull eccentricity in Duchenne muscular dystrophy. Journal of Child Neurology 2014;29(10):1344‐8. - PubMed
Tieleman 2009
-
- Tieleman AA, Knuijt S, Vliet J, Swart BJ, Ensink R, Engelen BG. Dysphagia is present but mild in myotonic dystrophy type 2. Neuromuscular Disorders 2009;19:196‐8. - PubMed
Willig 1994
-
- Willig TN, Paulus J, Lacau Saint Guily J, Béon C, Navarro J. Swallowing problems in neuromuscular disorders. Archives of Physical Medicine & Rehabilitation 1994;75(11):1175‐81. - PubMed
References to other published versions of this review
Hill 2002a
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials