Baseline Characteristics and Prescription Patterns of Standard Drugs in Patients with Angiographically Determined Coronary Artery Disease and Renal Failure (CAD-REF Registry)
- PMID: 26859890
- PMCID: PMC4747471
- DOI: 10.1371/journal.pone.0148057
Baseline Characteristics and Prescription Patterns of Standard Drugs in Patients with Angiographically Determined Coronary Artery Disease and Renal Failure (CAD-REF Registry)
Abstract
Background: Chronic kidney disease (CKD) is strongly associated with coronary artery disease (CAD). We established a prospective observational nationwide multicenter registry to evaluate current treatment and outcomes in patients with both CKD and angiographically documented CAD.
Methods: In 32 cardiological centers 3,352 CAD patients with ≥50% stenosis in at least one coronary artery were enrolled and classified according to their estimated glomerular filtration rate and proteinuria into one of five stages of CKD or as a control group.
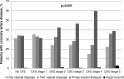
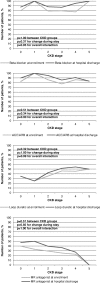
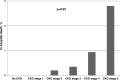
Results: 2,723 (81.2%) consecutively enrolled patients suffered from CKD. Compared to controls, CKD patients had a higher prevalence of diabetes, hypertension, peripheral artery diseases, heart failure, and valvular heart disease (each p<0.001). Myocardial infarctions (p = 0.02), coronary bypass grafting, valve replacements and pacemaker implantations had been recorded more frequently (each p<0.001). With advanced CKD, the number of diseased coronary vessels and the proportion of patients with reduced left ventricular ejection fraction (LVEF) increased significantly (both p<0.001). Percutaneous coronary interventions were performed less frequently (p<0.001) while coronary bypass grafting was recommended more often (p = 0.04) with advanced CKD. With regard to standard drugs in CAD treatment, prescriptions were higher in our registry than in previous reports, but beta-blockers (p = 0.008), and angiotensin-converting-enzyme inhibitors and/or angiotensin-receptor blockers (p<0.001) were given less often in higher CKD stages. In contrast, in the subgroup of patients with moderately to severely reduced LVEF the prescription rates did not differ between CKD stages. In-hospital mortality increased stepwise with each CKD stage (p = 0.02).
Conclusions: In line with other studies comprising CKD cohorts, patients' morbidity and in-hospital mortality increased with the degree of renal impairment. Although cardiologists' drug prescription rates in CAD-REF were higher than in previous studies, they were still lower especially in advanced CKD stages compared to cohorts treated by nephrologists.
Conflict of interest statement
Figures

Similar articles
-
Sex-specific differences and long-term outcome of patients with coronary artery disease and chronic kidney disease: the Coronary Artery Disease and Renal Failure (CAD-REF) Registry.Clin Res Cardiol. 2021 Oct;110(10):1625-1636. doi: 10.1007/s00392-021-01864-5. Epub 2021 May 26. Clin Res Cardiol. 2021. PMID: 34036426 Free PMC article.
-
The Coronary Artery Disease and Renal Failure (CAD-REF) registry: trial design, methods, and aims.Am Heart J. 2013 Sep;166(3):449-56. doi: 10.1016/j.ahj.2013.06.010. Epub 2013 Jul 23. Am Heart J. 2013. PMID: 24016493
-
Ventricular function and all-cause mortality in chronic kidney disease patients with angiographic coronary artery disease.J Nephrol. 2010 Mar-Apr;23(2):181-8. J Nephrol. 2010. PMID: 20119930
-
API expert consensus document on management of ischemic heart disease.J Assoc Physicians India. 2006 Jun;54:469-80. J Assoc Physicians India. 2006. PMID: 16909697 Review.
-
Coronary artery disease in patients with chronic kidney disease: a clinical update.Curr Cardiol Rev. 2013 Nov;9(4):331-9. doi: 10.2174/1573403x10666140214122234. Curr Cardiol Rev. 2013. PMID: 24527682 Free PMC article. Review.
Cited by
-
Association between renal function impairment and multivessel involvement in patients with acute ST-elevation myocardial infarction.Aging (Albany NY). 2020 May 20;12(11):10863-10872. doi: 10.18632/aging.103299. Epub 2020 May 20. Aging (Albany NY). 2020. PMID: 32433039 Free PMC article.
-
Living Lab Data of Patient Needs and Expectations for eHealth-Based Cardiac Rehabilitation in Germany and Spain From the TIMELY Study: Cross-Sectional Analysis.J Med Internet Res. 2024 Feb 22;26:e53991. doi: 10.2196/53991. J Med Internet Res. 2024. PMID: 38386376 Free PMC article.
-
Sex-specific differences and long-term outcome of patients with coronary artery disease and chronic kidney disease: the Coronary Artery Disease and Renal Failure (CAD-REF) Registry.Clin Res Cardiol. 2021 Oct;110(10):1625-1636. doi: 10.1007/s00392-021-01864-5. Epub 2021 May 26. Clin Res Cardiol. 2021. PMID: 34036426 Free PMC article.
-
ODM Data Analysis-A tool for the automatic validation, monitoring and generation of generic descriptive statistics of patient data.PLoS One. 2018 Jun 22;13(6):e0199242. doi: 10.1371/journal.pone.0199242. eCollection 2018. PLoS One. 2018. PMID: 29933373 Free PMC article.
-
[Anticoagulation in patients with chronic kidney disease : Recommendations from the working group "Heart-Kidney" of the German Cardiac Society and the German Society of Nephrology].Internist (Berl). 2017 May;58(5):512-521. doi: 10.1007/s00108-017-0220-5. Internist (Berl). 2017. PMID: 28396914 Review. German.
References
-
- Levey AS, Atkins R, Coresh J, Cohen EP, Collins AJ, Eckardt KU, et al. Chronic kidney disease as a global public health problem: approaches and initiatives—a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007;72:247–259. - PubMed
-
- Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154–2169. - PubMed
-
- Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

