Dynamic changes in the gene expression profile during rat oral carcinogenesis induced by 4-nitroquinoline 1-oxide
- PMID: 26860129
- PMCID: PMC4768982
- DOI: 10.3892/mmr.2016.4883
Dynamic changes in the gene expression profile during rat oral carcinogenesis induced by 4-nitroquinoline 1-oxide
Abstract
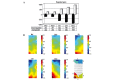
The typical progression of oral cancer is from hyperplastic epithelial lesions through dysplasia to invasive carcinoma. It is important to investigate malignant oral cancer progression and development in order to determine useful approaches of prevention of dysplastic lesions. The present study aimed to gain insights into the underlying molecular mechanism of oral carcinogenesis by establishing a rat model of oral carcinogenesis using 4‑nitroquinoline 1‑oxide. Subsequently, transcription profile analysis using an integrating microarray was performed. The dynamic gene expression changes of the six stages of rat oral carcinogenesis (normal, mild epithelial dysplasia, moderate dysplasia, severe dysplasia, carcinoma in situ and oral squamous cell carcinomas) were analyzed using component plane presentations (CPP)‑self‑organizing map (SOM). Six genes were verified by quantitative polymerase chain reaction, immunohistochemistry and succinate dehydrogenase (SDH) activity assay kit. Numerous differentially expressed genes (DEGs) were identified during rat oral carcinogenesis. CPP‑SOM determined that these DEGs were primarily enriched during cell cycle, apoptosis, inflammatory response and tricarboxylic acid cycle, indicating the coordinated regulation of molecular networks. In addition, the expression of specific DEGs, such as janus kinase 3, cyclin‑dependent kinase A‑1, B‑cell chronic lymphocytic leukaemia/lymphoma 2‑like 2, nuclear factor‑κB, tumor necrosis factor receptor superfamily member 1A, cyclin D1 and SDH were identified to have high concordance with the results from microarray data. The current study demonstrated that oral carcinogenesis is a multi‑step and multi‑gene process, with a distinct pattern alteration along a continuum of malignant transformation. In addition, this comprehensive investigation provided a theoretical basis for the understanding of the molecular alterations associated with oral carcinogenesis.
Figures




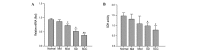
Similar articles
-
Oral-specific ablation of Klf4 disrupts epithelial terminal differentiation and increases premalignant lesions and carcinomas upon chemical carcinogenesis.J Oral Pathol Med. 2015 Nov;44(10):801-9. doi: 10.1111/jop.12307. Epub 2015 Jan 21. J Oral Pathol Med. 2015. PMID: 25605610
-
Abnormal expression of bcl-2 and bax in rat tongue mucosa during the development of squamous cell carcinoma induced by 4-nitroquinoline 1-oxide.Int J Exp Pathol. 2005 Dec;86(6):375-81. doi: 10.1111/j.0959-9673.2005.00444.x. Int J Exp Pathol. 2005. PMID: 16309543 Free PMC article.
-
[Expression of cytokeratin 19 and connexin 43 in 4-nitroquinoline-l-oxide-induced rat tongue carcinogenesis].Hua Xi Kou Qiang Yi Xue Za Zhi. 2013 Jun;31(3):237-41. Hua Xi Kou Qiang Yi Xue Za Zhi. 2013. PMID: 23841292 Chinese.
-
4-nitroquinoline-1-oxide induced experimental oral carcinogenesis.Oral Oncol. 2006 Aug;42(7):655-67. doi: 10.1016/j.oraloncology.2005.10.013. Epub 2006 Jan 30. Oral Oncol. 2006. PMID: 16448841 Review.
-
Experimental oral carcinogenesis. A basic rat model for the study of oral carcinogenesis using the carcinogen 4-nitroquinoline 1-oxide.Dan Med Bull. 1990 Oct;37(5):433-42. Dan Med Bull. 1990. PMID: 2125549 Review. No abstract available.
Cited by
-
Inhibition of Pro-inflammatory and Anti-apoptotic Biomarkers during Experimental Oral Cancer Chemoprevention by Dietary Black Raspberries.Front Immunol. 2017 Oct 23;8:1325. doi: 10.3389/fimmu.2017.01325. eCollection 2017. Front Immunol. 2017. PMID: 29109723 Free PMC article.
-
Characterization and Bio-Evaluation of the Synergistic Effect of Simvastatin and Folic Acid as Wound Dressings on the Healing Process.Pharmaceutics. 2023 Oct 4;15(10):2423. doi: 10.3390/pharmaceutics15102423. Pharmaceutics. 2023. PMID: 37896183 Free PMC article.
-
Yeast Ribonucleotide Reductase Is a Direct Target of the Proteasome and Provides Hyper Resistance to the Carcinogen 4-NQO.J Fungi (Basel). 2023 Mar 14;9(3):351. doi: 10.3390/jof9030351. J Fungi (Basel). 2023. PMID: 36983519 Free PMC article.
References
-
- Ferlay J, Bray F, Parkin DM, Pisani P, editors. Globocan 2000: Cancer Incidence and Mortality Worldwide. IARCPress; Lyon: 2001. (IARC Cancer Bases No. 5).
-
- Kademani D, Bell RB, Schmidt BL, Blanchaert R, Fernandes R, Lambert P, Tucker WM, American Association of Oral and Maxillofacial Surgeons Task Force on Oral Cancer A preliminary report from the American Association of Oral and Maxillofacial Surgeons Task Force on Oral Cancer. J Oral Maxillofac Surg. 2008;66:2151–2157. doi: 10.1016/j.joms.2008.06.030. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

