Ganglioside GD1a promotes oocyte maturation, furthers preimplantation development, and increases blastocyst quality in pigs
- PMID: 26860251
- PMCID: PMC4919288
- DOI: 10.1262/jrd.2015-083
Ganglioside GD1a promotes oocyte maturation, furthers preimplantation development, and increases blastocyst quality in pigs
Abstract
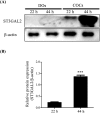
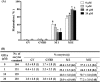
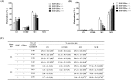
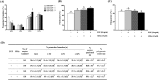
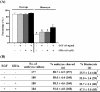
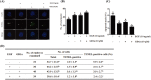
Gangliosides are key lipid molecules required for the regulation of cellular processes such as proliferation, differentiation, and cell signaling, including signaling of epidermal growth factor receptor (EGFR). Epidermal growth factor (EGF) has long been considered a potential regulator of meiotic and cytoplasmic maturation in mammalian oocytes. However, there is no report on the direct effect of ganglioside GD1a in porcine oocyte maturation. In this study, we first investigated a functional link between GD1a and meiotic maturation during in vitro maturation (IVM) of porcine embryos. Moreover, we confirmed the effect of exogenous GD1a treatment on blastocyst development, quality, and fertilization rate in early embryonic development. First, we observed that the protein level of ST3GAL2, a GD1a synthesizing enzyme, significantly increased (P < 0.01) in cumulus-oocyte-complexes (COCs) during IVM progress. The proportion of arrested germinal vesicles (GV) increased in oocytes treated with EGF+GD1a (41.6 ± 1.5%) at the IVM I stage. Upon completion of meiotic maturation, the proportion of metaphase II (M II) was significantly higher (P < 0.05) in the EGF+GD1a (89.9 ± 3.6%) treated group. After IVF, the percentage of penetrated oocytes was significantly higher (P < 0.05) in the EGF+GD1a (89.1 ± 2.3%) treated group than in the control group. Furthermore, exogenous GD1a treatment improved the developmental competence and quality of blastocysts during preimplantation embryo development stage. These results suggest that ganglioside GD1a may play an important role in IVM mechanisms of porcine maturation capacity. Furthermore, our findings will be helpful for better promoting the embryo development and blastocyst quality in pigs.
Figures
Similar articles
-
A pre-in vitro maturation medium containing cumulus oocyte complex ligand-receptor signaling molecules maintains meiotic arrest, supports the cumulus oocyte complex and improves oocyte developmental competence.Mol Hum Reprod. 2017 Sep 1;23(9):594-606. doi: 10.1093/molehr/gax032. Mol Hum Reprod. 2017. PMID: 28586460
-
Effects of combined epidermal growth factor, brain-derived neurotrophic factor and insulin-like growth factor-1 on human oocyte maturation and early fertilized and cloned embryo development.Hum Reprod. 2012 Jul;27(7):2146-59. doi: 10.1093/humrep/des099. Epub 2012 Apr 23. Hum Reprod. 2012. PMID: 22532606
-
An improved IVM method for cumulus-oocyte complexes from small follicles in polycystic ovary syndrome patients enhances oocyte competence and embryo yield.Hum Reprod. 2017 Oct 1;32(10):2056-2068. doi: 10.1093/humrep/dex262. Hum Reprod. 2017. PMID: 28938744
-
Porcine oocyte maturation in vitro: role of cAMP and oocyte-secreted factors - A practical approach.J Reprod Dev. 2016 Oct 18;62(5):439-449. doi: 10.1262/jrd.2016-016. Epub 2016 Jun 24. J Reprod Dev. 2016. PMID: 27349308 Free PMC article. Review.
-
Effects of Gangliosides on Spermatozoa, Oocytes, and Preimplantation Embryos.Int J Mol Sci. 2019 Dec 22;21(1):106. doi: 10.3390/ijms21010106. Int J Mol Sci. 2019. PMID: 31877897 Free PMC article. Review.
Cited by
-
Lipid Signaling During Gamete Maturation.Front Cell Dev Biol. 2022 Jun 24;10:814876. doi: 10.3389/fcell.2022.814876. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36204680 Free PMC article. Review.
-
Lipid Stores and Lipid Metabolism Associated Gene Expression in Porcine and Bovine Parthenogenetic Embryos Revealed by Fluorescent Staining and RNA-seq.Int J Mol Sci. 2020 Sep 5;21(18):6488. doi: 10.3390/ijms21186488. Int J Mol Sci. 2020. PMID: 32899450 Free PMC article.
-
The Association Between Follicular Fluid Sialic Acid Levels, Oocyte Quality, and Pregnancy Rates.Reprod Sci. 2022 Feb;29(2):633-638. doi: 10.1007/s43032-021-00688-y. Epub 2021 Jul 15. Reprod Sci. 2022. PMID: 34264515
-
The Molecular Regulation in the Pathophysiology in Ovarian Aging.Aging Dis. 2021 Jun 1;12(3):934-949. doi: 10.14336/AD.2020.1113. eCollection 2021 Jun. Aging Dis. 2021. PMID: 34094652 Free PMC article. Review.
-
Regulation of the Endoplasmic Reticulum Stress by BIP/GRP78 is involved in Meiotic Maturation of Porcine Oocytes In Vitro.Dev Reprod. 2017 Dec;21(4):407-415. doi: 10.12717/DR.2017.21.4.407. Epub 2017 Dec 31. Dev Reprod. 2017. PMID: 29354786 Free PMC article.
References
-
- Kim SM, Jung JU, Ryu JS, Jin JW, Yang HJ, Ko K, You HK, Jung KY, Choo YK. Effects of gangliosides on the differentiation of human mesenchymal stem cells into osteoblasts by modulating epidermal growth factor receptors. Biochem Biophys Res Commun 2008; 371: 866–871. - PubMed
-
- Xu Y, Tan LJ, Grachtchouk V, Voorhees JJ, Fisher GJ. Receptor-type protein-tyrosine phosphatase-kappa regulates epidermal growth factor receptor function. J Biol Chem 2005; 280: 42694–42700. - PubMed
-
- Scaltriti M, Baselga J. The epidermal growth factor receptor pathway: a model for targeted therapy. Clin Cancer Res 2006; 12: 5268–5272. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

