The diagnostic accuracy of the MTBDRplus and MTBDRsl assays for drug-resistant TB detection when performed on sputum and culture isolates
- PMID: 26860462
- PMCID: PMC4748215
- DOI: 10.1038/srep17850
The diagnostic accuracy of the MTBDRplus and MTBDRsl assays for drug-resistant TB detection when performed on sputum and culture isolates
Abstract
Although molecular tests for drug-resistant TB perform well on culture isolates, their accuracy using clinical samples, particularly from TB and HIV-endemic settings, requires clarification. The MTBDRplus and MTBDRsl line probe assays were evaluated in 181 sputum samples and 270 isolates from patients with culture-confirmed drug-sensitive-TB, MDR-TB, or XDR-TB. Phenotypic culture-based testing was the reference standard. Using sputum, the sensitivities for resistance was 97.7%, 95.4%, 58.9%, 61.6% for rifampicin, isoniazid, ofloxacin, and amikacin, respectively, whereas the specificities were 91.8%, 89%, 100%, and 100%, respectively. MTBDRsl sensitivity differed in smear-positive vs. smear-negative samples (79.2% vs. 20%, p < 0.0001 for ofloxacin; 72.9% vs. 37%, p = 0.0023 for amikacin) but not by HIV status. If used sequentially, MTBDRplus and MTBDRsl could rule-in XDR-TB in 78.5% (22/28) and 10.5% (2/19) of smear-positive and smear-negative samples, respectively. On culture isolates, the sensitivity for resistance to rifampicin, isoniazid, ofloxacin, and amikacin was 95.1%, 96.1%, 72.3% and 76.6%, respectively, whereas the specificities exceeded 96%. Using a sequential testing approach, rapid sputum-based diagnosis of fluoroquinolone or aminoglycoside-resistant TB is feasible only in smear-positive samples, where rule-in value is good. Further investigation is required in samples that test susceptible in order to rule-out second-line drug resistance.
Figures
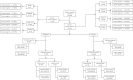
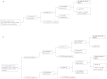
Similar articles
-
GenoType® MTBDRsl assay for resistance to second-line anti-tuberculosis drugs.Cochrane Database Syst Rev. 2016 Sep 8;9(9):CD010705. doi: 10.1002/14651858.CD010705.pub3. Cochrane Database Syst Rev. 2016. PMID: 27605387 Free PMC article. Review.
-
Rapid detection of extensively drug-resistant (XDR-TB) strains from multidrug-resistant tuberculosis (MDR-TB) cases isolated from smear-negative pulmonary samples in an Intermediate Reference Laboratory in India.Indian J Tuberc. 2016 Jul;63(3):144-148. doi: 10.1016/j.ijtb.2016.07.009. Indian J Tuberc. 2016. PMID: 27865234
-
Performance of the MTBDRsl assay in Georgia.Int J Tuberc Lung Dis. 2014 Feb;18(2):233-9. doi: 10.5588/ijtld.13.0468. Int J Tuberc Lung Dis. 2014. PMID: 24429319 Free PMC article.
-
Direct detection of resistance to fluoroquinolones/SLIDs in sputum specimen by GenoType MTBDRsl v.2.0 assay A study from Eastern Uttar Pradesh, India.Ann Clin Microbiol Antimicrob. 2021 Aug 26;20(1):56. doi: 10.1186/s12941-021-00463-6. Ann Clin Microbiol Antimicrob. 2021. PMID: 34446022 Free PMC article.
-
Evaluation of genotype MTBDRplus VER 2.0 line probe assay for the detection of MDR-TB in smear positive and negative sputum samples.BMC Infect Dis. 2017 Apr 17;17(1):280. doi: 10.1186/s12879-017-2389-6. BMC Infect Dis. 2017. PMID: 28415989 Free PMC article.
Cited by
-
Rapid Molecular Assays for the Diagnosis of Drug-Resistant Tuberculosis.Infect Drug Resist. 2022 Aug 29;15:4971-4984. doi: 10.2147/IDR.S381643. eCollection 2022. Infect Drug Resist. 2022. PMID: 36060232 Free PMC article. Review.
-
Combined Locked Nucleic Acid Probes and High-Resolution Melting Curve Analysis for Detection of Rifampicin-Resistant Tuberculosis in Northern Thailand.Diagnostics (Basel). 2022 Sep 24;12(10):2307. doi: 10.3390/diagnostics12102307. Diagnostics (Basel). 2022. PMID: 36291996 Free PMC article.
-
Evaluation of extracts from used Xpert MTB/RIF cartridges for detection of resistance to second-line anti-tuberculosis drugs in patients with multidrug-resistant tuberculosis in Ethiopia.BMC Microbiol. 2025 Jan 17;25(1):26. doi: 10.1186/s12866-025-03746-6. BMC Microbiol. 2025. PMID: 39825226 Free PMC article.
-
GenoType® MTBDRsl assay for resistance to second-line anti-tuberculosis drugs.Cochrane Database Syst Rev. 2016 Sep 8;9(9):CD010705. doi: 10.1002/14651858.CD010705.pub3. Cochrane Database Syst Rev. 2016. PMID: 27605387 Free PMC article. Review.
-
Association of gyrA and rrs gene mutations detected by MTBDRsl V1 on Mycobacterium tuberculosis strains of diverse genetic background from India.Sci Rep. 2018 Jun 18;8(1):9295. doi: 10.1038/s41598-018-27299-z. Sci Rep. 2018. PMID: 29915257 Free PMC article.
References
-
- World Health Organisation. Tuberculosis Fact Sheet Number 104 (2014). Available at: www.who.int/mediacentre/factsheets/fs104/en (Accessed: 5th June 2014).
-
- World Health Organization. WHO Global Task Force outlines measures to combat XDR-TB worldwide (2014). Available at: http://www.who.int/mediacentre/news/notes/2006/np29/en/ (Accessed on: 5th June 2014).
-
- Centers for Disease Control and Prevention. Emergence of Mycobacterium tuberculosis with extensive resistance to second-line drugs worldwide, 2000–2004. MMWR Morb Mortal Wkly Rep. 55, 301–305 (2006). - PubMed
-
- Dheda K. et al. Early treatment outcomes and HIV status of patients with extensively drug-resistant tuberculosis in South Africa: a retrospective cohort study. Lancet. 375, 1798–1807 (2010). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

