Long-term clinical outcomes of everolimus-eluting stent versus paclitaxel-eluting stent in patients undergoing percutaneous coronary interventions: a meta-analysis
- PMID: 26860585
- PMCID: PMC4748592
- DOI: 10.1186/s12872-016-0206-6
Long-term clinical outcomes of everolimus-eluting stent versus paclitaxel-eluting stent in patients undergoing percutaneous coronary interventions: a meta-analysis
Abstract
Background: Everolimus -eluting stent (EES) is common used in patients undergoing percutaneous coronary interventions (PCI). Our purpose is to evaluate long-term clinical outcomes of everolimus -eluting stent (EES) versus paclitaxel-eluting stent (PES) in patients undergoing percutaneous coronaryinterventions (PCI) in randomized controlled trials (RCTs).
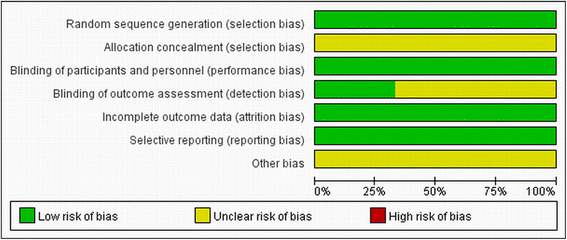
Methods: We searched Medline, EMBASE, Cochrane Library, CNKI, VIP and relevant websites ( https://scholar-google-com.ezproxy.lib.usf.edu/ ) for articles to compare outcomes between everolimus-eluting stent and paclitaxel-eluting stent without language or date restriction. RCTs that compared the use of everolimus -eluting stent and paclitaxel-eluting stent in PCI were included. Variables relating to patient, study characteristics, and clinical endpoints were extracted. Meta-analysis was performed using RevMan 5.2 software.
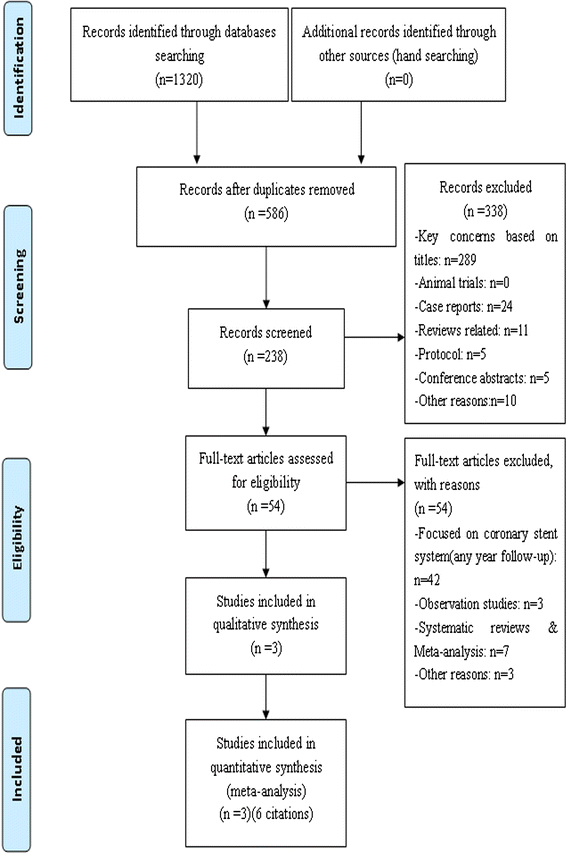
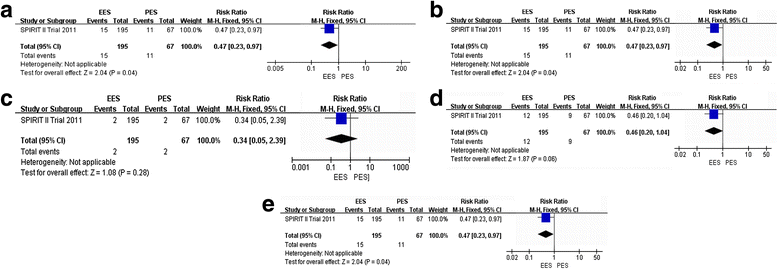
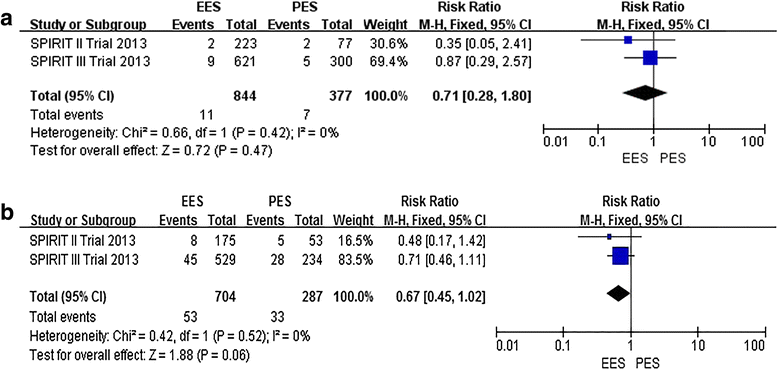
Results: We identified 6 published studies (from three randomized trials) more on everolimus-eluting stent (n = 3352) than paclitaxel-eluting (n = 1639), with follow-up duration ranging from 3, 4 and 5 years. Three-year outcomes of everolimus-eluting stent compared to paclitaxel-eluting were as following: the everolimus-eluting stent significantly reduced all-cause death (relative risk [RR]:0.63; 95% confidence interval [CI]: 0.46. to 0.82), MACE (RR: 0.56; 95% CI: 0.41 to 0.77), MI (RR: 0.64; 95% CI: 0.48 to 0.86), TLR (RR: 0.72; 95% CI: 0.59 to 0.88), ID-TLR (RR: 0.74; 95% CI: 0.59 to 0.92) and ST (RR: 0.54; 95% CI: 0.32 to 0.90). There was no difference in TVR between the everolimus-eluting and paclitaxel-eluting (RR: 0.76; 95% CI: 0.58 to 1.10); Four-year outcomes of everolimus-eluting compared to paclitaxel-eluting: the everolimus-eluting significantly reduced MACE (RR: 0.44; 95% CI: 0.18 to 0.98) and ID-TLR (RR: 0.47; 95 % CI: 0.23 to 0.97). There was no difference in MI (RR: 0.48; 95% CI: 0.16 to 1.46), TLR (RR: 0.46; 95% CI: 0.20 to 1.04) and ST ((RR: 0.34; 95% CI: 0.05 to 2.39). Five-year outcomes of everolimus-eluting stent compared to paclitaxel-eluting: There was no difference in ID-TLR (RR: 0.67; 95% CI: 0.45 to 1.02) and ST (RR: 0.71; 95% CI: 0.28 to 1.80).
Conclusions: In the present meta-analysis, everolimus-eluting appeared to be safe and clinically effective in patients undergoing PCI in comparison to PES in 3-year clinical outcomes; there was similar no difference in reduction of ST between EES and PES in long-term(≥ 4 years) clinical follow-ups. Everolimus-eluting is more safety than paclitaxel-eluting in long-term clinical follow-ups, whether these effects can be applied to different patient subgroups warrants further investigation.
Figures


Similar articles
-
Meta-analysis of everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease: final 3-year results of the SPIRIT clinical trials program (Clinical Evaluation of the Xience V Everolimus Eluting Coronary Stent System in the Treatment of Patients With De Novo Native Coronary Artery Lesions).JACC Cardiovasc Interv. 2013 Sep;6(9):914-22. doi: 10.1016/j.jcin.2013.05.005. JACC Cardiovasc Interv. 2013. PMID: 24050859
-
5-year results of a randomized comparison of XIENCE V everolimus-eluting and TAXUS paclitaxel-eluting stents: final results from the SPIRIT III trial (clinical evaluation of the XIENCE V everolimus eluting coronary stent system in the treatment of patients with de novo native coronary artery lesions).JACC Cardiovasc Interv. 2013 Dec;6(12):1263-6. doi: 10.1016/j.jcin.2013.07.009. Epub 2013 Nov 13. JACC Cardiovasc Interv. 2013. PMID: 24239202 Clinical Trial.
-
Everolimus-eluting stents versus sirolimus- or paclitaxel-eluting stents: two-year results from the Guthrie Health Off-Label Stent (GHOST) registry.J Interv Cardiol. 2013 Apr;26(2):153-62. doi: 10.1111/j.1540-8183.2013.12016.x. Epub 2013 Jan 31. J Interv Cardiol. 2013. PMID: 23363439
-
Long time clinical outcomes of limus-eluting stent versus paclitaxel-eluting stent in patients undergoing percutaneous coronary artery intervention: A meta-analysis of randomized controlled clinical trials.Cardiol J. 2014;21(3):211-9. doi: 10.5603/CJ.a2014.0004. Epub 2014 Feb 14. Cardiol J. 2014. PMID: 24526500 Review.
-
Efficacy and safety of everolimus and zotarolimus-eluting stents versus first-generation drug-eluting stents in patients with diabetes: A meta-analysis of randomized trials.Int J Cardiol. 2017 Mar 1;230:310-318. doi: 10.1016/j.ijcard.2016.12.116. Epub 2016 Dec 27. Int J Cardiol. 2017. PMID: 28062139 Review.
Cited by
-
Mass Spectrometry Imaging of Biomaterials.Materials (Basel). 2023 Sep 21;16(18):6343. doi: 10.3390/ma16186343. Materials (Basel). 2023. PMID: 37763619 Free PMC article. Review.
-
A comparison of the main outcomes from BP-BES and DP-DES at five years of follow-up: A systematic review and meta-analysis.Sci Rep. 2017 Nov 3;7(1):14997. doi: 10.1038/s41598-017-14247-6. Sci Rep. 2017. PMID: 29101374 Free PMC article.
-
Efficacy and safety of nicorandil on perioperative myocardial injury in patients undergoing elective percutaneous coronary intervention: results of the PENMIPCI trial.Drug Des Devel Ther. 2018 Aug 22;12:2591-2599. doi: 10.2147/DDDT.S173931. eCollection 2018. Drug Des Devel Ther. 2018. PMID: 30174413 Free PMC article. Clinical Trial.
-
Safety and Efficacy of an Innovative Everolimus-Coated Balloon in a Swine Coronary Artery Model.Life (Basel). 2023 Oct 13;13(10):2053. doi: 10.3390/life13102053. Life (Basel). 2023. PMID: 37895434 Free PMC article.
-
Association study of matrix metalloproteinase 3 5A/6A polymorphism with in-stent restenosis after percutaneous coronary interventions in a Han Chinese population.J Int Med Res. 2020 Jan;48(1):300060519827145. doi: 10.1177/0300060519827145. Epub 2019 Feb 8. J Int Med Res. 2020. PMID: 30732526 Free PMC article.
References
-
- Menzin J, Wygant G, Hauch O et al. One-year costs of ischemic heart disease among patients with acute coronary syndromes: findings from a multi-employer claims database*. Curr. Med. Res. Opin.® 2008; 24: 461–468. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

