Mast cell-derived neurotrophin 4 mediates allergen-induced airway hyperinnervation in early life
- PMID: 26860818
- PMCID: PMC4980297
- DOI: 10.1038/mi.2016.11
Mast cell-derived neurotrophin 4 mediates allergen-induced airway hyperinnervation in early life
Abstract
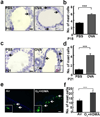
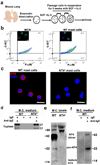
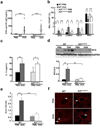
Asthma often progresses from early episodes of insults. How early-life events connect to long-term airway dysfunction remains poorly understood. We demonstrated previously that increased neurotrophin 4 (NT4) levels following early-life allergen exposure cause persistent changes in airway smooth muscle (ASM) innervation and airway hyper-reactivity (AHR) in mice. Herein, we identify pulmonary mast cells as a key source of aberrant NT4 expression following early insults. NT4 is selectively expressed by ASM and mast cells in mice, nonhuman primates, and humans. We show in mice that mast cell-derived NT4 is dispensable for ASM innervation during development. However, upon insults, mast cells expand in number and degranulate to release NT4 and thus become the major source of NT4 under pathological condition. Adoptive transfer of wild-type mast cells, but not NT4-/- mast cells restores ASM hyperinnervation and AHR in KitW-sh/W-sh mice following early-life insults. Notably, an infant nonhuman primate model of asthma also exhibits ASM hyperinnervation associated with the expansion and degranulation of mast cells. Together, these findings identify an essential role of mast cells in mediating ASM hyperinnervation following early-life insults by producing NT4. This role may be evolutionarily conserved in linking early insults to long-term airway dysfunction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
An NT4/TrkB-dependent increase in innervation links early-life allergen exposure to persistent airway hyperreactivity.FASEB J. 2014 Feb;28(2):897-907. doi: 10.1096/fj.13-238212. Epub 2013 Nov 12. FASEB J. 2014. PMID: 24221086 Free PMC article.
-
Role of mast cells in airway remodeling.Curr Opin Immunol. 2007 Dec;19(6):687-93. doi: 10.1016/j.coi.2007.07.018. Epub 2007 Aug 29. Curr Opin Immunol. 2007. PMID: 17761410 Review.
-
IL-33 drives airway hyper-responsiveness through IL-13-mediated mast cell: airway smooth muscle crosstalk.Allergy. 2015 May;70(5):556-67. doi: 10.1111/all.12593. Epub 2015 Mar 16. Allergy. 2015. PMID: 25683166 Free PMC article.
-
Mast cell-derived TNF contributes to airway hyperreactivity, inflammation, and TH2 cytokine production in an asthma model in mice.J Allergy Clin Immunol. 2007 Jul;120(1):48-55. doi: 10.1016/j.jaci.2007.02.046. Epub 2007 May 7. J Allergy Clin Immunol. 2007. PMID: 17482668
-
The role of the mast cell in asthma: induction of airway hyperresponsiveness by interaction with smooth muscle?J Allergy Clin Immunol. 2004 Jul;114(1):58-65. doi: 10.1016/j.jaci.2004.03.034. J Allergy Clin Immunol. 2004. PMID: 15241345 Review.
Cited by
-
Cytokines and beyond: Regulation of innate immune responses during helminth infection.Cytokine. 2020 Sep;133:154527. doi: 10.1016/j.cyto.2018.08.021. Epub 2018 Sep 18. Cytokine. 2020. PMID: 30241895 Free PMC article. Review.
-
Neuroimmune regulatory networks of the airway mucosa in allergic inflammatory disease.J Leukoc Biol. 2022 Jan;111(1):209-221. doi: 10.1002/JLB.3RU0121-023R. Epub 2021 Apr 15. J Leukoc Biol. 2022. PMID: 33857344 Free PMC article. Review.
-
Sex-specific airway hyperreactivity and sex-specific transcriptome remodeling in neonatal piglets challenged with intra-airway acid.Am J Physiol Lung Cell Mol Physiol. 2019 Jan 1;316(1):L131-L143. doi: 10.1152/ajplung.00417.2018. Epub 2018 Nov 8. Am J Physiol Lung Cell Mol Physiol. 2019. PMID: 30407862 Free PMC article.
-
Unique Allergic Asthma Phenotypes in Offspring of House Dust Mite-exposed Mice.Am J Respir Cell Mol Biol. 2022 Jul;67(1):89-98. doi: 10.1165/rcmb.2021-0535OC. Am J Respir Cell Mol Biol. 2022. PMID: 35363997 Free PMC article.
-
Targeting acetylcholine receptor M3 prevents the progression of airway hyperreactivity in a mouse model of childhood asthma.FASEB J. 2017 Oct;31(10):4335-4346. doi: 10.1096/fj.201700186R. Epub 2017 Jun 15. FASEB J. 2017. PMID: 28619712 Free PMC article.
References
-
- Maddox L, Schwartz DA. The pathophysiology of asthma. Annu. Rev. Med. 2002;53:477–498. - PubMed
-
- Dakhama A, et al. The enhancement or prevention of airway hyperresponsiveness during reinfection with respiratory syncytial virus is critically dependent on the age at first infection and IL-13 production. J. Immunol. 2005;175:1876–1883. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

