Activation of zebrafish Src family kinases by the prion protein is an amyloid-β-sensitive signal that prevents the endocytosis and degradation of E-cadherin/β-catenin complexes in vivo
- PMID: 26860872
- PMCID: PMC4748561
- DOI: 10.1186/s13024-016-0076-5
Activation of zebrafish Src family kinases by the prion protein is an amyloid-β-sensitive signal that prevents the endocytosis and degradation of E-cadherin/β-catenin complexes in vivo
Abstract
Background: Prions and amyloid-β (Aβ) oligomers trigger neurodegeneration by hijacking a poorly understood cellular signal mediated by the prion protein (PrP) at the plasma membrane. In early zebrafish embryos, PrP-1-dependent signals control cell-cell adhesion via a tyrosine phosphorylation-dependent mechanism.
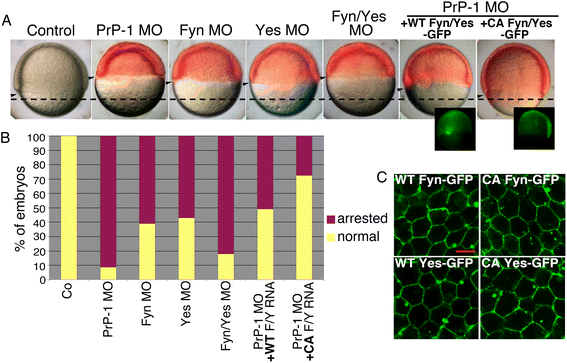
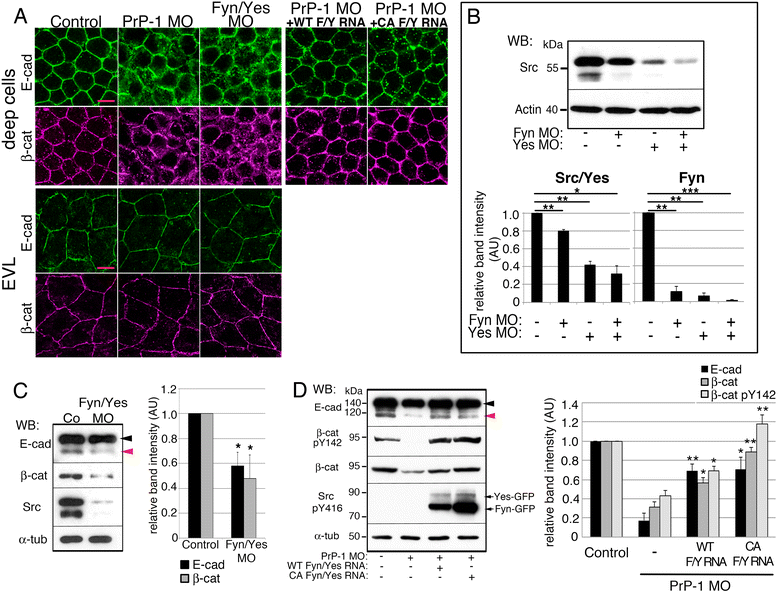
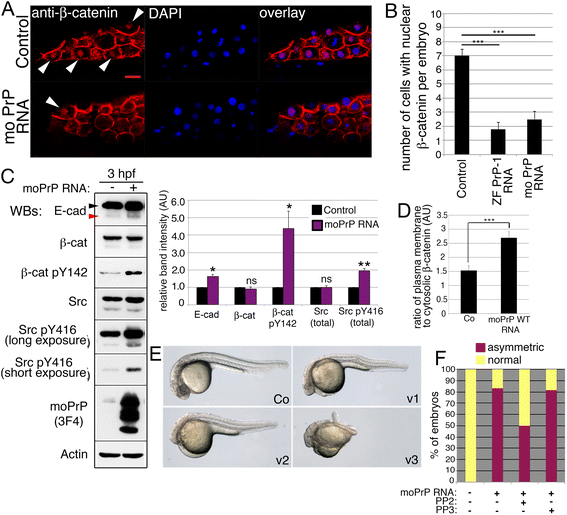
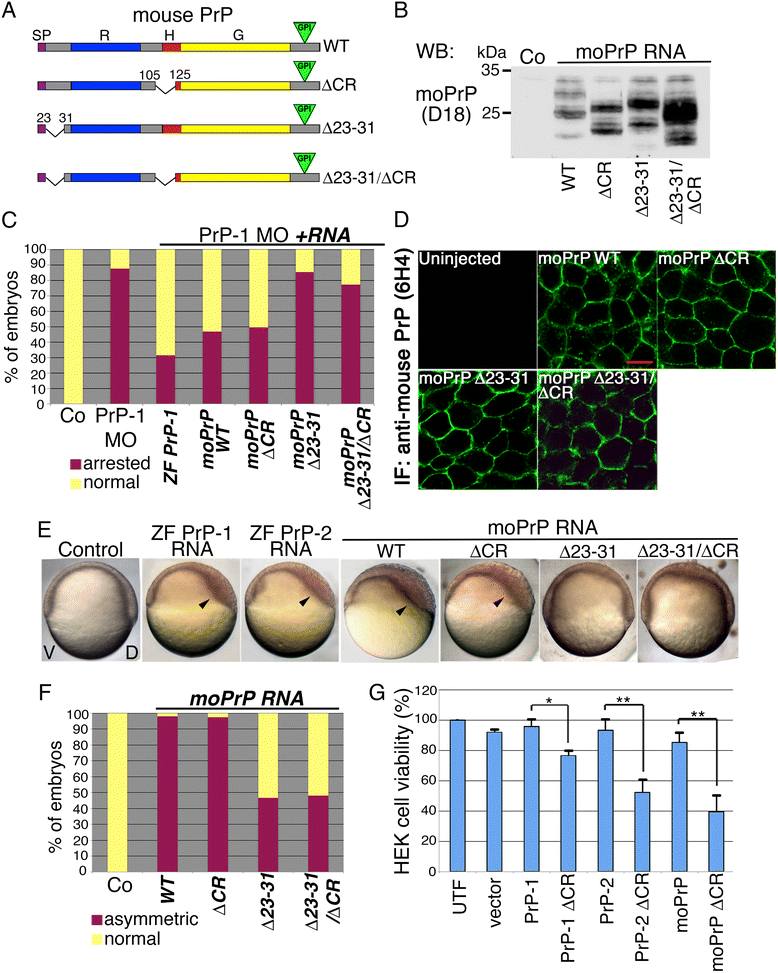
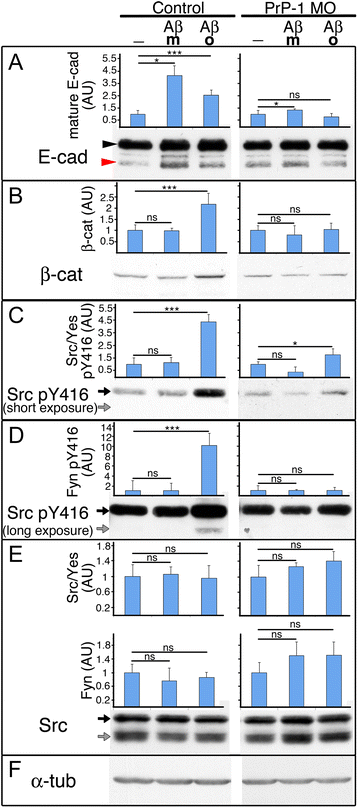
Results: Here we report that the Src family kinases (SFKs) Fyn and Yes act downstream of PrP-1 to prevent the endocytosis and degradation of E-cadherin/β-catenin adhesion complexes in vivo. Accordingly, knockdown of PrP-1 or Fyn/Yes cause similar zebrafish gastrulation phenotypes, whereas Fyn/Yes expression rescues the PrP-1 knockdown phenotype. We also show that zebrafish and mouse PrPs positively regulate the activity of Src kinases and that these have an unexpected positive effect on E-cadherin-mediated cell adhesion. Interestingly, while PrP knockdown impairs β-catenin adhesive function, PrP overexpression enhances it, thereby antagonizing its nuclear, wnt-related signaling activity and disturbing embryonic dorsoventral specification. The ability of mouse PrP to influence these events in zebrafish embryos requires its neuroprotective, polybasic N-terminus but not its neurotoxicity-associated central region. Remarkably, human Aβ oligomers up-regulate the PrP-1/SFK/E-cadherin/β-catenin pathway in zebrafish embryonic cells, mimicking a PrP gain-of-function scenario.
Conclusions: Our gain- and loss-of-function experiments in zebrafish suggest that PrP and SFKs enhance the cell surface stability of embryonic adherens junctions via the same complex mechanism through which they over-activate neuroreceptors that trigger synaptic damage. The profound impact of this pathway on early zebrafish development makes these embryos an ideal model to study the cellular and molecular events affected by neurotoxic PrP mutations and ligands in vivo. In particular, our finding that human Aβ oligomers activate the zebrafish PrP/SFK/E-cadherin pathway opens the possibility of using fish embryos to rapidly screen for novel therapeutic targets and compounds against prion- and Alzheimer's-related neurodegeneration. Altogether, our data illustrate PrP-dependent signals relevant to embryonic development, neuronal physiology and neurological disease.
Figures



Similar articles
-
Uncontrolled SFK-mediated protein trafficking in prion and Alzheimer's disease.Prion. 2016 Sep 2;10(5):352-361. doi: 10.1080/19336896.2016.1221873. Prion. 2016. PMID: 27649856 Free PMC article.
-
Regulation of embryonic cell adhesion by the prion protein.PLoS Biol. 2009 Mar 10;7(3):e55. doi: 10.1371/journal.pbio.1000055. PLoS Biol. 2009. PMID: 19278297 Free PMC article.
-
Common themes in PrP signaling: the Src remains the same.Front Cell Dev Biol. 2014 Oct 28;2:63. doi: 10.3389/fcell.2014.00063. eCollection 2014. Front Cell Dev Biol. 2014. PMID: 25364767 Free PMC article.
-
PrPs: Proteins with a purpose: Lessons from the zebrafish.Prion. 2009 Jul-Sep;3(3):129-33. doi: 10.4161/pri.3.3.9651. Epub 2009 Jul 29. Prion. 2009. PMID: 19786844 Free PMC article. Review.
-
Cellular prion protein: A co-receptor mediating neuronal cofilin-actin rod formation induced by β-amyloid and proinflammatory cytokines.Prion. 2014;8(6):375-80. doi: 10.4161/pri.35504. Prion. 2014. PMID: 25426519 Free PMC article. Review.
Cited by
-
Uncontrolled SFK-mediated protein trafficking in prion and Alzheimer's disease.Prion. 2016 Sep 2;10(5):352-361. doi: 10.1080/19336896.2016.1221873. Prion. 2016. PMID: 27649856 Free PMC article.
-
Transcriptomic analysis of zebrafish prion protein mutants supports conserved cross-species function of the cellular prion protein.Prion. 2021 Dec;15(1):70-81. doi: 10.1080/19336896.2021.1924557. Prion. 2021. PMID: 34139950 Free PMC article.
-
Wnt/β-catenin signaling regulates VE-cadherin-mediated anastomosis of brain capillaries by counteracting S1pr1 signaling.Nat Commun. 2018 Nov 19;9(1):4860. doi: 10.1038/s41467-018-07302-x. Nat Commun. 2018. PMID: 30451830 Free PMC article.
-
Expression of engrailed homeobox 2 regulates the proliferation, migration and invasion of non-small cell lung cancer cells.Oncol Lett. 2018 Jul;16(1):536-542. doi: 10.3892/ol.2018.8693. Epub 2018 May 10. Oncol Lett. 2018. PMID: 29963129 Free PMC article.
-
Physiological Functions of the Cellular Prion Protein.Front Mol Biosci. 2017 Apr 6;4:19. doi: 10.3389/fmolb.2017.00019. eCollection 2017. Front Mol Biosci. 2017. PMID: 28428956 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

