Percutaneous Transluminal Angioplasty and Drug-Eluting Stents for Infrapopliteal Lesions in Critical Limb Ischemia (PADI) Trial
- PMID: 26861113
- PMCID: PMC4753788
- DOI: 10.1161/CIRCINTERVENTIONS.114.002376
Percutaneous Transluminal Angioplasty and Drug-Eluting Stents for Infrapopliteal Lesions in Critical Limb Ischemia (PADI) Trial
Abstract
Background: Endovascular infrapopliteal treatment of patients with critical limb ischemia using percutaneous transluminal angioplasty (PTA) and bail-out bare metal stenting (BMS) is hampered by restenosis. In interventional cardiology, drug-eluting stents (DES) have shown better patency rates and are standard practice nowadays. An investigator-initiated, multicenter, randomized trial was conducted to assess whether DES also improve patency and clinical outcome of infrapopliteal lesions.
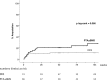
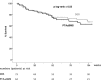
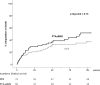
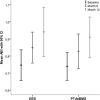
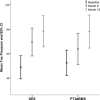
Methods and results: Adults with critical limb ischemia (Rutherford category ≥4) and infrapopliteal lesions were randomized to receive PTA±BMS or DES with paclitaxel. Primary end point was 6-month primary binary patency of treated lesions, defined as ≤50% stenosis on computed tomographic angiography. Stenosis >50%, retreatment, major amputation, and critical limb ischemia-related death were regarded as treatment failure. Severity of failure was assessed with an ordinal score, ranging from vessel stenosis through occlusion to the clinical failures. Seventy-four limbs (73 patients) were treated with DES and 66 limbs (64 patients) received PTA±BMS. Six-month patency rates were 48.0% for DES and 35.1% for PTA±BMS (P=0.096) in the modified-intention-to-treat and 51.9% and 35.1% (P=0.037) in the per-protocol analysis. The ordinal score showed significantly worse treatment failure for PTA±BMS versus DES (P=0.041). The observed major amputation rate remained lower in the DES group until 2 years post-treatment, with a trend toward significance (P=0.066). Less minor amputations occurred after DES until 6 months post-treatment (P=0.03).
Conclusions: In patients with critical limb ischemia caused by infrapopliteal lesions, DES provide better 6-month patency rates and less amputations after 6 and 12 months compared with PTA±BMS.
Clinical trial registration: URL: http://www.clinicaltrials.gov. Unique identifier: NCT00471289.
Keywords: angioplasty; drug-eluting stents; ischemia; peripheral arterial disease.
© 2016 The Authors.
Figures


References
-
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG TASC II Working Group. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. 2007;45(suppl S):S5–S67. doi: 10.1016/j.jvs.2006.12.037. - PubMed
-
- Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM, Jr, White CJ, White J, White RA, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B American Association for Vascular Surgery/Society for Vascular Surgery; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society of Interventional Radiology; ACC/AHA Task Force on Practice Guidelines. ACC/AHA guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Associations for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease)–summary of recommendations. J Vasc Interv Radiol. 2006;17:1383–1397, quiz 1398. doi: 10.1097/01.RVI.0000240426.53079.46. - PubMed
-
- Adam DJ, Beard JD, Cleveland T, Bell J, Bradbury AW, Forbes JF, Fowkes FG, Gillepsie I, Ruckley CV, Raab G, Storkey H BASIL Trial Participants. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. 2005;366:1925–1934. doi: 10.1016/S0140-6736(05)67704-5. - PubMed
-
- Nehler MR, Coll JR, Hiatt WR, Regensteiner JG, Schnickel GT, Klenke WA, Strecker PK, Anderson MW, Jones DN, Whitehill TA, Moskowitz S, Krupski WC. Functional outcome in a contemporary series of major lower extremity amputations. J Vasc Surg. 2003;38:7–14. - PubMed
-
- van Overhagen H, Spiliopoulos S, Tsetis D. Below-the-knee interventions. Cardiovasc Intervent Radiol. 2013;36:302–311. doi: 10.1007/s00270-013-0550-1. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

