Immunologic response in treatment-naïve HIV-2-infected patients: the IeDEA West Africa cohort
- PMID: 26861115
- PMCID: PMC4748109
- DOI: 10.7448/IAS.19.1.20044
Immunologic response in treatment-naïve HIV-2-infected patients: the IeDEA West Africa cohort
Abstract
Introduction: Response to antiretroviral therapy (ART) among individuals infected with HIV-2 is poorly described. We compared the immunological response among patients treated with three nucleoside reverse-transcriptase inhibitors (NRTIs) to boosted protease inhibitor (PI) and unboosted PI-based regimens in West Africa.
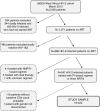
Methods: This prospective cohort study enrolled treatment-naïve HIV-2-infected patients within the International Epidemiological Databases to Evaluate AIDS collaboration in West Africa. We used mixed models to compare the CD4 count response to treatment over 12 months between regimens.
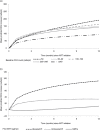
Results: Of 422 HIV-2-infected patients, 285 (67.5%) were treated with a boosted PI-based regimen, 104 (24.6%) with an unboosted PI-based regimen and 33 (7.8%) with three NRTIs. Treatment groups were comparable with regard to gender (54.5% female) and median age at ART initiation (45.3 years; interquartile range 38.3 to 51.8). Treatment groups differed by clinical stage (21.2%, 16.8% and 17.3% at CDC Stage C or World Health Organization Stage IV for the triple NRTI, boosted PI and unboosted PI groups, respectively, p=0.02), median length of follow-up (12.9, 17.7 and 44.0 months for the triple NRTI, the boosted PI and the unboosted PI groups, respectively, p<0.001) and baseline median CD4 count (192, 173 and 129 cells/µl in the triple NRTI, the boosted PI and the unboosted PI-based regimen groups, respectively, p=0.003). CD4 count recovery at 12 months was higher for patients treated with boosted PI-based regimens than those treated with three NRTIs or with unboosted PI-based regimens (191 cells/µl, 95% CI 142 to 241; 110 cells/µl, 95% CI 29 to 192; 133 cells/µl, 95% CI 80 to 186, respectively, p=0.004).
Conclusions: In this observational study using African data, boosted PI-containing regimens had better immunological response compared to triple NRTI combinations and unboosted PI-based regimens at 12 months. A randomized clinical trial is still required to determine the best initial regimen for treating HIV-2 infected patients.
Keywords: HIV-2; West Africa; antiretroviral treatment; immunological response; linear mixed models.
Figures

References
-
- UNAIDS. UNAIDS report on the global AIDS epidemic 2006. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS (UNAIDS); 2006. [cited 2014 Feb 24]; Available from: http://data.unaids.org/pub/epireport/2006/2006_epiupdate_en.pdf.
-
- van der Loeff MF, Awasana AA, Sarge-Njie R, van der Sande M, Jaye A, Sabally S, et al. Sixteen years of HIV surveillance in a West African research clinic reveals divergent epidemic trends of HIV-1 and HIV-2. Int J Epidemiol. 2006;35:1322–8. - PubMed
-
- Eholie S, Anglaret X. Commentary: decline of HIV-2 prevalence in West Africa: good news or bad news? Int J Epidemiol. 2006;35:1329–30. - PubMed
-
- Valadas E, Franca L, Sousa S, Antunes F. 20 years of HIV-2 infection in Portugal: trends and changes in epidemiology. Clin Infect Dis. 2009;48:1166–7. - PubMed
-
- da Silva Z, Oliveira I, Andersen A, Dias F, Rodrigues A, Holmgren B, et al. Changes in prevalence and incidence of HIV-1, HIV-2 and dual infections in urban areas of Bissau, Guinea-Bissau: is HIV-2 disappearing? AIDS. 2008;22:1195–202. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

