Focus on Extracellular Vesicles: Introducing the Next Small Big Thing
- PMID: 26861301
- PMCID: PMC4783904
- DOI: 10.3390/ijms17020170
Focus on Extracellular Vesicles: Introducing the Next Small Big Thing
Abstract
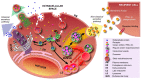
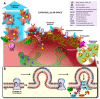
Intercellular communication was long thought to be regulated exclusively through direct contact between cells or via release of soluble molecules that transmit the signal by binding to a suitable receptor on the target cell, and/or via uptake into that cell. With the discovery of small secreted vesicular structures that contain complex cargo, both in their lumen and the lipid membrane that surrounds them, a new frontier of signal transduction was discovered. These "extracellular vesicles" (EV) were initially thought to be garbage bags through which the cell ejected its waste. Whilst this is a major function of one type of EV, i.e., apoptotic bodies, many EVs have intricate functions in intercellular communication and compound exchange; although their physiological roles are still ill-defined. Additionally, it is now becoming increasingly clear that EVs mediate disease progression and therefore studying EVs has ignited significant interests among researchers from various fields of life sciences. Consequently, the research effort into the pathogenic roles of EVs is significantly higher even though their protective roles are not well established. The "Focus on extracellular vesicles" series of reviews highlights the current state of the art regarding various topics in EV research, whilst this review serves as an introductory overview of EVs, their biogenesis and molecular composition.
Keywords: apoptotic body; biogenesis; ectosome; exosome; extracellular vesicle; isolation; microvesicle; molecular composition; signal transduction.
Figures



References
-
- Cossetti C., Iraci N., Mercer T.R., Leonardi T., Alpi E., Drago D., Alfaro-Cervello C., Saini H.K., Davis M.P., Schaeffer J. Extracellular vesicles from neural stem cells transfer IFN-γ via Ifngr1 to activate Stat1 signaling in target cells. Mol. Cell. 2014;56:193–204. doi: 10.1016/j.molcel.2014.08.020. - DOI - PMC - PubMed
-
- Théry C., Zitvogel L., Amigorena S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002;2:569–579. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

