Clinical Trial of Oral Nelfinavir before and during Radiation Therapy for Advanced Rectal Cancer
- PMID: 26861457
- PMCID: PMC4835023
- DOI: 10.1158/1078-0432.CCR-15-1489
Clinical Trial of Oral Nelfinavir before and during Radiation Therapy for Advanced Rectal Cancer
Abstract
Purpose: Nelfinavir, a PI3K pathway inhibitor, is a radiosensitizer that increases tumor blood flow in preclinical models. We conducted an early-phase study to demonstrate the safety of nelfinavir combined with hypofractionated radiotherapy (RT) and to develop biomarkers of tumor perfusion and radiosensitization for this combinatorial approach.
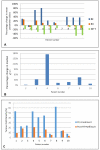
Experimental design: Ten patients with T3-4 N0-2 M1 rectal cancer received 7 days of oral nelfinavir (1,250 mg b.i.d.) and a further 7 days of nelfinavir during pelvic RT (25 Gy/5 fractions/7 days). Perfusion CT (p-CT) and DCE-MRI scans were performed pretreatment, after 7 days of nelfinavir and prior to the last fraction of RT. Biopsies taken pretreatment and 7 days after the last fraction of RT were analyzed for tumor cell density (TCD).
Results: There were 3 drug-related grade 3 adverse events: diarrhea, rash, and lymphopenia. On DCE-MRI, there was a mean 42% increase in medianKtrans, and a corresponding median 30% increase in mean blood flow on p-CT during RT in combination with nelfinavir. Median TCD decreased from 24.3% at baseline to 9.2% in biopsies taken 7 days after RT (P= 0.01). Overall, 5 of 9 evaluable patients exhibited good tumor regression on MRI assessed by tumor regression grade (mrTRG).
Conclusions: This is the first study to evaluate nelfinavir in combination with RT without concurrent chemotherapy. It has shown that nelfinavir-RT is well tolerated and is associated with increased blood flow to rectal tumors. The efficacy of nelfinavir-RT versus RT alone merits clinical evaluation, including measurement of tumor blood flow.
©2016 American Association for Cancer Research.
Figures

References
-
- Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–46. - PubMed
-
- Theodoropoulos G, Wise WE, Padmanabhan A, Kerner BA, Taylor CW, Aguilar PS, et al. T-level downstaging and complete pathologic response after preoperative chemoradiation for advanced rectal cancer result in decreased recurrence and improved disease-free survival. Dis Colon Rectum. 2002;45:895–903. - PubMed
-
- Bouzourene H, Bosman FT, Seelentag W, Matter M, Coucke P. Importance of tumor regression assessment in predicting the outcome in patients with locally advanced rectal carcinoma who are treated with preoperative radiotherapy. Cancer. 2002;94:1121–30. - PubMed
-
- Vironen J, Juhola M, Kairaluoma M, Jantunen I, Kellokumpu I. Tumour regression grading in the evaluation of tumour response after different preoperative radiotherapy treatments for rectal carcinoma. Int J Colorectal Dis. 2005;20:440–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

