Treatment of Adults with Lennox-Gastaut Syndrome: Further Analysis of Efficacy and Safety/Tolerability of Rufinamide
- PMID: 26861566
- PMCID: PMC4919131
- DOI: 10.1007/s40120-016-0041-9
Treatment of Adults with Lennox-Gastaut Syndrome: Further Analysis of Efficacy and Safety/Tolerability of Rufinamide
Abstract
Introduction: Management of Lennox-Gastaut syndrome (LGS) in adulthood can be particularly challenging. Published reports describing the use of rufinamide specifically in adult patients with LGS are scarce. A post hoc subgroup analysis of data from a phase III trial was conducted to investigate the efficacy and safety/tolerability of rufinamide in adults with LGS.
Methods: A randomized, double-blind, placebo-controlled trial was conducted in patients with LGS, aged 4 years and above. During an 84-day, double-blind treatment period, patients received either adjunctive rufinamide therapy or placebo. Efficacy and safety/tolerability were assessed in a post hoc subgroup analysis of adult patients (≥18 years). Efficacy was assessed as change from baseline in 28-day seizure frequency, 50% responder rate, and seizure freedom rate; each calculated for total seizures and drop attacks. Safety/tolerability assessments included the evaluation of adverse events (AEs).
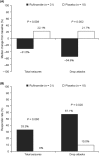
Results: Thirty-one adults aged 18-37 years with LGS received treatment with either rufinamide (n = 21) or placebo (n = 10). Three patients in the rufinamide group did not complete the trial. The median change from baseline in seizure frequency was -31.5% for rufinamide versus +22.1% for placebo (P = 0.008) for all seizures and -54.9% versus +21.7% (P = 0.002) for drop attacks. Responder rates were 33.3% for rufinamide versus 0% for placebo (P = 0.066) for all seizures and 57.1% versus 10.0% (P = 0.020) for drop attacks. No patient achieved freedom from all seizures but two rufinamide-treated patients (9.5%) became free of drop attacks. Overall, 71.4% of patients treated with rufinamide and 60.0% of patients treated with placebo experienced AEs; most commonly, somnolence (33.3% vs. 20.0%) and vomiting (19.0% vs. 0%). Most AEs were of mild or moderate intensity.
Conclusion: Rufinamide demonstrated favorable efficacy and was generally well tolerated when used as adjunctive treatment for adults with LGS.
Funding: Eisai.
Keywords: Adult; Antiepileptic drug; Epilepsy; Lennox–Gastaut syndrome; Rufinamide.
Figures
Similar articles
-
Rufinamide as an adjunctive therapy for Lennox-Gastaut syndrome: a randomized double-blind placebo-controlled trial in Japan.Epilepsy Res. 2014 Nov;108(9):1627-36. doi: 10.1016/j.eplepsyres.2014.08.019. Epub 2014 Sep 2. Epilepsy Res. 2014. PMID: 25219353 Clinical Trial.
-
Safety and efficacy of rufinamide in children and adults with Lennox-Gastaut syndrome: A post hoc analysis from Study 022.Epilepsy Behav. 2021 Sep 9;124:108275. doi: 10.1016/j.yebeh.2021.108275. Epub 2021 Sep 9. Epilepsy Behav. 2021. PMID: 34509883
-
Rufinamide: a pharmacoeconomic profile of its use as adjunctive therapy in Lennox-Gastaut syndrome.Pharmacoeconomics. 2012 Mar;30(3):247-56. doi: 10.2165/11208630-000000000-00000. Pharmacoeconomics. 2012. PMID: 22332960 Review.
-
Role of rufinamide in the management of Lennox-Gastaut syndrome (childhood epileptic encephalopathy).Neuropsychiatr Dis Treat. 2007 Feb;3(1):3-11. doi: 10.2147/nedt.2007.3.1.3. Neuropsychiatr Dis Treat. 2007. PMID: 19300535 Free PMC article.
-
Efficacy and safety of rufinamide as adjunctive therapy in patients with Lennox Gastaut syndrome: A systematic review and Meta-analysis.Seizure. 2021 Oct;91:296-307. doi: 10.1016/j.seizure.2021.07.004. Epub 2021 Jul 9. Seizure. 2021. PMID: 34273668
Cited by
-
Rufinamide add-on therapy for drug-resistant epilepsy.Cochrane Database Syst Rev. 2020 Nov 8;11(11):CD011772. doi: 10.1002/14651858.CD011772.pub3. Cochrane Database Syst Rev. 2020. PMID: 33179247 Free PMC article.
-
Movement disorders associated with antiseizure medications: A systematic review.Epilepsy Behav. 2022 Jun;131(Pt A):108693. doi: 10.1016/j.yebeh.2022.108693. Epub 2022 Apr 25. Epilepsy Behav. 2022. PMID: 35483204 Free PMC article.
-
Expert Opinion on the Management of Lennox-Gastaut Syndrome: Treatment Algorithms and Practical Considerations.Front Neurol. 2017 Sep 29;8:505. doi: 10.3389/fneur.2017.00505. eCollection 2017. Front Neurol. 2017. PMID: 29085326 Free PMC article. Review.
-
Anti-seizure medications for Lennox-Gastaut syndrome.Cochrane Database Syst Rev. 2021 Apr 7;4(4):CD003277. doi: 10.1002/14651858.CD003277.pub4. Cochrane Database Syst Rev. 2021. PMID: 33825230 Free PMC article.
-
Rufinamide add-on therapy for refractory epilepsy.Cochrane Database Syst Rev. 2018 Apr 25;4(4):CD011772. doi: 10.1002/14651858.CD011772.pub2. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2020 Nov 8;11:CD011772. doi: 10.1002/14651858.CD011772.pub3. PMID: 29691835 Free PMC article. Updated.
References
-
- Arzimanoglou A, Resnick T. All children who experience epileptic falls do not necessarily have Lennox–Gastaut syndrome… but many do. Epileptic Disord. 2011;13(Suppl 1):S3–S13. - PubMed
-
- Kerr M, Kluger G, Philip S. Evolution and management of Lennox–Gastaut syndrome through adolescence and into adulthood: are seizures always the primary issue? Epileptic Disord. 2011;13(Suppl 1):S15–S26. - PubMed
-
- Resnick T, Arzimanoglou A, Brown LW, et al. Rufinamide from clinical trials to clinical practice in the United States and Europe. Epileptic Disord. 2011;13(Suppl 1):S27–S43. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources