Efficacy of Intraoperative Dexmedetomidine Compared with Placebo for Postoperative Pain Management: A Meta-Analysis of Published Studies
- PMID: 26861737
- PMCID: PMC4912966
- DOI: 10.1007/s40122-016-0045-2
Efficacy of Intraoperative Dexmedetomidine Compared with Placebo for Postoperative Pain Management: A Meta-Analysis of Published Studies
Abstract
Introduction: Dexmedetomidine (Dex) has sedative, analgesic, and anesthetic-sparing effects. This meta-analysis examines demonstrated intraoperative and postoperative effects of intraoperative Dex administration during pediatric surgery.
Methods: A search for randomized placebo-controlled trials was conducted to identify clinical trials examining intraoperative Dex use in children, infants, and neonates. Primary outcome was postoperative opioid consumption; secondary outcomes were: postoperative pain intensity and postoperative nausea and vomiting (PONV).
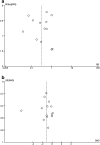
Results: Fourteen randomized controlled trials performed during painful procedures were analyzed. Intraoperative Dex administration was associated with significantly reduced postoperative opioid consumption in the postanesthesia care unit [PACU; risk ratio (RR) = 0.31 (0.17, 0.59), I (2) = 76%, p < 0.0001 and cumulative z score using trial sequential analysis], decreased pain intensity in PACU [standardized mean difference (SMD) = -1.18 (-1.88, -0.48), I (2) = 91%, p < 0.0001] but had no effect upon PONV incidence [RR = 0.67 (0.41, 1.08), I (2) = 0%, p = 0.48]. Subgroup analyses found administering Dex during adenotonsillectomy and using a bolus <0.5 µg/kg (irrespective to the use of a continuous administration) without effects on studies outcomes. Heterogeneity was high among results and a high suspicion of publication bias was present for all analyzed outcomes.
Conclusions: This meta-analysis shows that intraoperative Dex administration in children reduces postoperative opioids consumption and postoperative pain in PACU. According to our results, optimal bolus dose was found to be ≥0.5 µg/kg. Future studies have to explore this particular point and the postoperative analgesic effects of Dex during longer periods.
Keywords: Analgesia; Children; Dexmedetomidine; Meta-analysis; Postoperative pain; Recovery.
Figures




References
-
- Barr J, Fraser GL, Puntillo K, Ely EW, Gélinas C, Dasta JF, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263–306. - PubMed
-
- Le Bot A, Michelet D, Hilly J, Maesani M, Dilly MP, Brasher C, et al. Efficacy of intraoperative dexmedetomidine compared with placebo for surgery in adults: a meta-analysis of published studies. Minerva Anestesiol. 2015;81(10):1105–1117. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

