Transient Expression of Fez Family Zinc Finger 2 Protein Regulates the Brn3b Gene in Developing Retinal Ganglion Cells
- PMID: 26861874
- PMCID: PMC4817192
- DOI: 10.1074/jbc.M115.689448
Transient Expression of Fez Family Zinc Finger 2 Protein Regulates the Brn3b Gene in Developing Retinal Ganglion Cells
Abstract
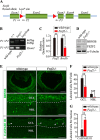
Retinal ganglion cells (RGCs) are projection neurons in the neural retina that relay visual information from the environment to the central nervous system. The early expression of MATH5 endows the post-mitotic precursors with RGC competence and leads to the activation ofBrn3bthat marks committed RGCs. Nevertheless, this fate commitment process and, specifically, regulation ofBrn3bremain elusive. To explore the molecular mechanisms underlying RGC generation in the mouse retina, we analyzed the expression and function of Fez family zinc finger 2 (FEZF2), a transcription factor critical for the development of projection neurons in the cerebral cortex.Fezf2mRNA and protein were transiently expressed at embryonic day 16.5 in the inner neuroblast layer and the prospective ganglion cell layer of the retina, respectively. Knockout ofFezf2in the developing retina reduced BRN3B+ cells and increased apoptotic cell markers.Fezf2knockdown by retinalin uteroelectroporation diminished BRN3B but not the coexpressed ISLET1 and BRN3A, indicating that the BRN3B decrease was the cause, not the result, of the overall reduction of BRN3B+ RGCs in theFezf2knockout retina. Moreover, the mRNA and promoter activity ofBrn3bwere increasedin vitroby FEZF2, which bound to a 5' regulatory fragment in theBrn3bgenomic locus. These results indicate that transient expression ofFezf2in the retina modulates the transcription ofBrn3band the survival of RGCs. This study improves our understanding of the transcriptional cascade required for the specification of RGCs and provides novel insights into the molecular basis of retinal development.
Keywords: Brn3b; Fezf2; gene expression; gene knockout; in utero electroporation; mouse; neurodevelopment; retinal ganglion cells; transcription regulation.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures






References
-
- Yang Z., Ding K., Pan L., Deng M., and Gan L. (2003) Math5 determines the competence state of retinal ganglion cell progenitors. Dev. Biol. 264, 240–254 - PubMed
-
- Hutcheson D. A., and Vetter M. L. (2001) The bHLH factors Xath5 and XNeuroD can upregulate the expression of XBrn3d, a POU-homeodomain transcription factor. Dev. Biol. 232, 327–338 - PubMed
-
- Gan L., Wang S. W., Huang Z., and Klein W. H. (1999) POU domain factor Brn-3b is essential for retinal ganglion cell differentiation and survival but not for initial cell fate specification. Dev. Biol. 210, 469–480 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

