Antithrombin III for critically ill patients: a systematic review with meta-analysis and trial sequential analysis
- PMID: 26862016
- PMCID: PMC7095103
- DOI: 10.1007/s00134-016-4225-7
Antithrombin III for critically ill patients: a systematic review with meta-analysis and trial sequential analysis
Abstract
Purpose: Antithrombin III (AT III) is an anticoagulant with anti-inflammatory properties. We assessed the benefits and harms of AT III in critically ill patients.
Methods: We searched from inception to 27 August 2015 in CENTRAL, MEDLINE, EMBASE, CAB, BIOSIS and CINAHL. We included randomized controlled trials (RCTs) irrespective of publication status, date of publication, blinding status, outcomes published or language.
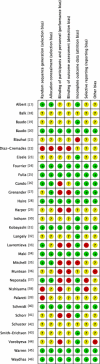
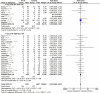
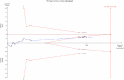
Results: We included 30 RCTs with a total of 3933 participants. The majority of included trials were at high risk of bias. Combining all trials, regardless of bias, showed no statistically significant effect of AT III on mortality (RR 0.95, 95% CI 0.88-1.03, I (2) = 0%, fixed-effect model, 29 trials, 3882 participants). Among those with severe sepsis and disseminated intravascular coagulation (DIC), AT III showed no impact on mortality (RR 0.95, 95% Cl 0.88-1.03, I (2) = 0%, fixed-effect model, 12 trials, 2858 participants). We carried out multiple subgroup and sensitivity analyses to assess the benefits and harms of AT III and to examine the impact of risk of bias. AT III significantly increased bleeding events (RR 1.58, 95% CI 1.35-1.84, I (2) = 0%, fixed-effect model, 11 trials, 3019 participants). However, for all other outcome measures and analyses, the results did not reach statistical significance.
Conclusions: There is insufficient evidence to support AT III substitution in any category of critically ill participants including those with sepsis and DIC. AT III did not show an impact on mortality, but increased the risk of bleeding.
Keywords: Antithrombin III; Bleeding; DIC; Multi organ failure; Sepsis; Septic shock.
Conflict of interest statement
Mikkel Allingstrup, Frederikke B. Ravn, Ann Merete Møller and Arash Afshari declare that there are no conflicts of interest. Jørn Wetterslev declares that he is a member of the task force on TSA at Copenhagen Trial Unit developing and programming TSA.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

