Angiotensin-converting enzyme 2 activator diminazene aceturate prevents lipopolysaccharide-induced inflammation by inhibiting MAPK and NF-κB pathways in human retinal pigment epithelium
- PMID: 26862037
- PMCID: PMC4748536
- DOI: 10.1186/s12974-016-0489-7
Angiotensin-converting enzyme 2 activator diminazene aceturate prevents lipopolysaccharide-induced inflammation by inhibiting MAPK and NF-κB pathways in human retinal pigment epithelium
Abstract
Background: Retinal inflammation is a devastating pathological process in ocular diseases. Functional impairment of retinal pigment epithelium (RPE) is associated with inflammatory retinal diseases. Enhancing the protective axis namely ACE2/Ang-(1-7)/Mas by activation of ACE2 presents anti-inflammatory properties. We investigated whether diminazene aceturate (DIZE), an angiotensin-converting enzyme 2 (ACE2) activator, prevented lipopolysaccharide (LPS)-induced inflammatory response by activating the protective axis and whether the effect was mediated by inhibiting the mitogen-activated protein kinase (MAPK) and the nuclear factor-κB (NF-κB) pathways.
Methods: Cell counting kit-8 (CCK-8) assay and real-time PCR were used to determine the optimum concentration and incubation time of DIZE. ARPE-19 cells and primary cultured human retinal pigment epithelia (hRPE) were incubated with or without 10 μg/mL DIZE for 6 h before stimulated with 5 μg/mL LPS for 24 h. The mRNA expression of inflammatory cytokines, AT1R, and AT2R was analyzed. The protein level of inflammatory cytokines, Ang II, and Ang-(1-7) was detected. Phosphorylation of p38 MAPK, extracellular signal-regulated kinase (ERK)1/2, c-Jun N-terminal kinase (JNK) and phosphorylated transcription inhibition factor-κB-α (p-IκB-α) were measured. Inhibitors of MAPKs and NF-κB were added to verify the involvement of these pathways. A small interfering RNA (siRNA) targeted to ACE2 and a selective Ang-(1-7) antagonist A779 was used to confirm the role of ACE2 and the involvement of ACE2/Ang-(1-7)/Mas axis.
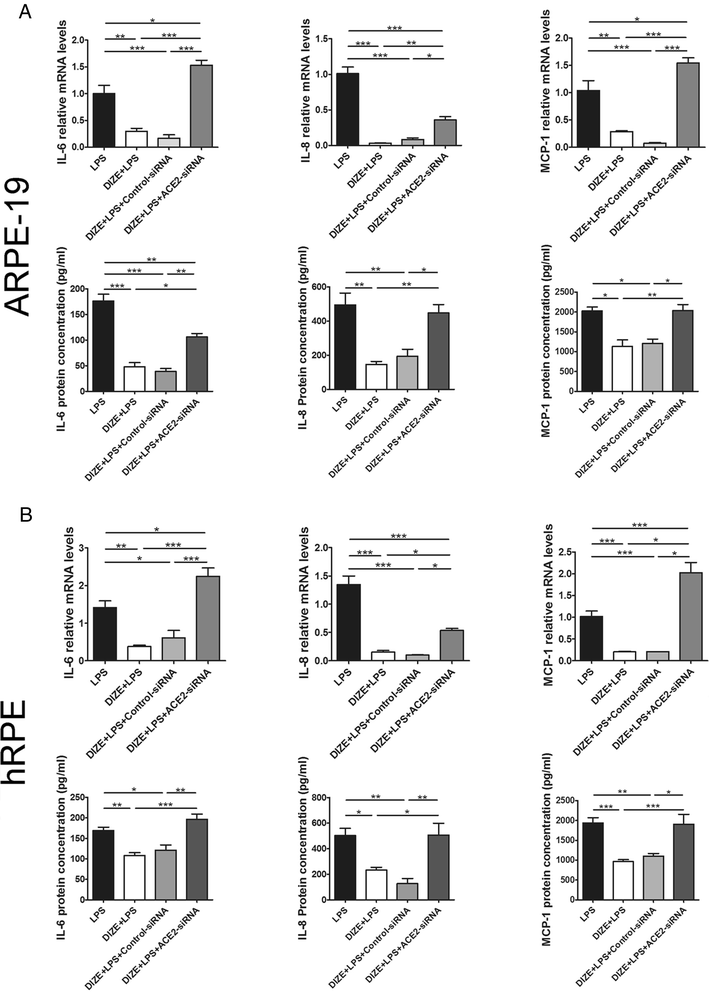
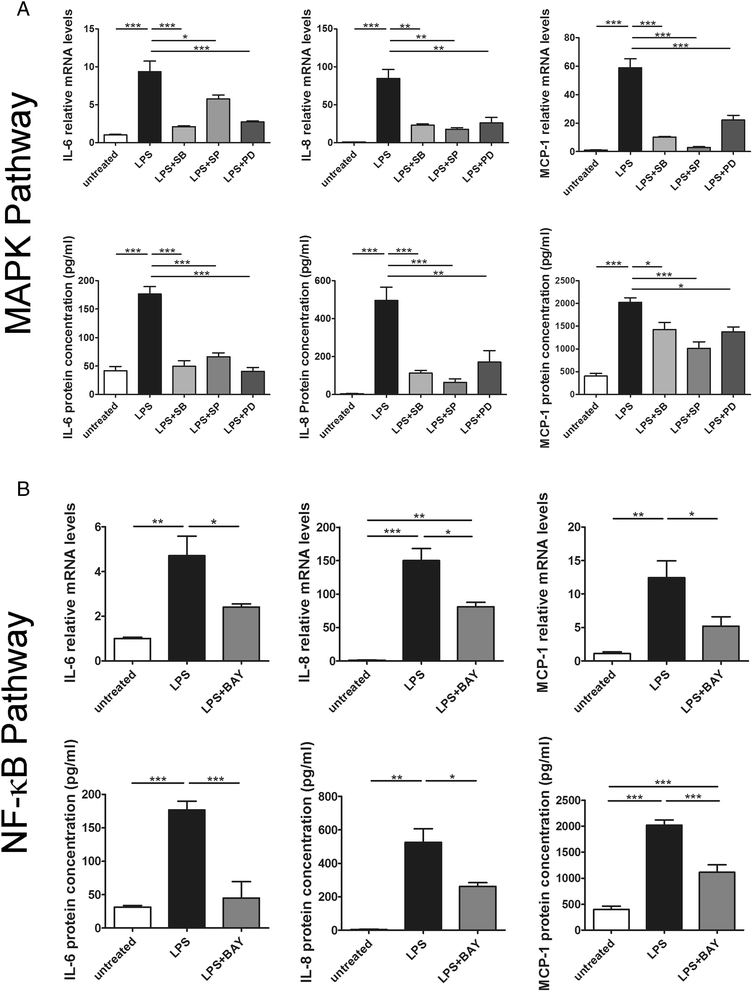
Results: DIZE remarkably increased the expression of ACE2 and inhibited the expression of IL-6, IL-8, and MCP-1 at both mRNA and protein levels in both RPE cell lines stimulated with LPS. Inhibitors of p38, ERK1/2, JNK, and NF-κB significantly decreased LPS-induced overproduction of IL-6, IL-8, and MCP-1. DIZE reduced the expression of Ang II and AT1R, whereas increased Ang-(1-7). Furthermore, DIZE downregulated the phosphorylation of p38MAPK, ERK1/2, JNK, and the activation of NF-κB upon stimulation with LPS. Downregulating ACE2 and pre-treatment with A779 abrogated the effects of DIZE on production of cytokines, the expression of Ang II, Ang-(1-7), AT1R, phosphorylation of MAPKs and activation of NF-κB.
Conclusions: DIZE inhibits LPS-induced inflammatory response by activating ACE2/Ang-(1-7)/Mas axis in human RPE cells. The protective effect is mediated by inhibiting the p38MAPK, ERK1/2, JNK, and NF-κB pathways.
Figures










References
-
- van Thiel BS, van der Pluijm I, Te Riet L, Essers J, Danser AH. The renin-angiotensin system and its involvement in vascular disease. European journal of pharmacology. 2015. doi:10.1016/j.ejphar.2015.03.090. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

