The Irish National Adverse Events Study (INAES): the frequency and nature of adverse events in Irish hospitals-a retrospective record review study
- PMID: 26862223
- PMCID: PMC5284341
- DOI: 10.1136/bmjqs-2015-004828
The Irish National Adverse Events Study (INAES): the frequency and nature of adverse events in Irish hospitals-a retrospective record review study
Abstract
Introduction: Irish healthcare has undergone extensive change recently with spending cuts and a focus on quality initiatives; however, little is known about adverse event occurrence.
Objective: To assess the frequency and nature of adverse events in Irish hospitals.
Methods: 1574 (53% women, mean age 54 years) randomly selected adult inpatient admissions from a sample of eight hospitals, stratified by region and size, across the Republic of Ireland in 2009 were reviewed using two-stage (nurse review of patient charts, followed by physician review of triggered charts) retrospective chart review with electronic data capture. Results were weighted to reflect the sampling strategy. The impact on adverse event rate of differing application of international adverse event criteria was also examined.
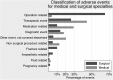
Results: 45% of charts were triggered. The prevalence of adverse events in admissions was 12.2% (95% CI 9.5% to 15.5%), with an incidence of 10.3 events per 100 admissions (95% CI 7.5 to 13.1). Over 70% of events were considered preventable. Two-thirds were rated as having a mild-to-moderate impact on the patient, 9.9% causing permanent impairment and 6.7% contributing to death. A mean of 6.1 added bed days was attributed to events, representing an expenditure of €5550 per event. The adverse event rate varied substantially (8.6%-17.0%) when applying different published adverse event eligibility criteria.
Conclusions: This first study of adverse events in Ireland reports similar rates to other countries. In a time of austerity, adverse events in adult inpatients were estimated to cost over €194 million. These results provide important baseline data on the adverse event burden and, alongside web-based chart review, provide an incentive and methodology to monitor future patient-safety initiatives.
Keywords: Adverse events, epidemiology and detection; Chart review methodologies; Patient safety; Trigger tools.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures

References
-
- Wilson RM, Runciman WB, Gibberd RW, et al. The quality in Australian health care study. Med J Aust 1995;163:458.–. - PubMed
-
- Health Information and Quality Authority. Investigation into the safety, quality and standards of services provided by the Health Service Executive to patients, including pregnant women, at risk of clinical deterioration, including those provided in University Hospital Galway, and as reflected in the care and treatment provided to Savita Halappanavar. 7 October 2013 (cited 4 November 2015). http://www.hiqa.ie/system/files/Patient-Safety-Investigation-UHG-Summary...
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
