Costs of Expanded Rapid HIV Testing in Four Emergency Departments
- PMID: 26862232
- PMCID: PMC4720608
- DOI: 10.1177/00333549161310S109
Costs of Expanded Rapid HIV Testing in Four Emergency Departments
Abstract
Objective: The HIV Prevention Trials Network (HPTN) 065 trial sought to expand HIV screening of emergency department (ED) patients in Bronx, New York, and Washington, D.C. This study assessed the testing costs associated with different expansion processes and compared them with costs of a hypothetical optimized process.
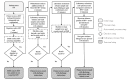
Methods: Micro-costing studies were conducted in two participating EDs in each city that switched from point-of-care (POC) to rapid-result laboratory testing. In three EDs, laboratory HIV testing was only conducted for patients having blood drawn for clinical reasons; in the other ED, all HIV testing was conducted with laboratory testing. Costs were estimated through direct observation and interviews to document process flows, time estimates, and labor and materials costs. A hypothetical optimized process flow used minimum time estimates for each process step. National wage and fringe rates and local reagent costs were used to determine the average cost (excluding overhead) per completed nonreactive and reactive test in 2013 U.S. dollars.
Results: Laboratory HIV testing costs in the EDs ranged from $17.00 to $23.83 per completed nonreactive test, and POC testing costs ranged from $17.64 to $37.60; cost per completed reactive test ranged from $89.29 to $123.17. Costs of hypothetical optimized HIV testing with automated process steps were approximately 45% lower for nonreactive tests and 20% lower for reactive tests. The cost per ED visit to conduct expanded HIV testing in each hospital ranged from $1.21 to $3.96.
Conclusion: An optimized process could achieve additional cost savings but would require an investment in electronic system interfaces to further automate testing processes.
Figures
References
-
- Centers for Disease Control and Prevention (US) HIV Surveillance Supplemental Report. No. 3. Vol. 19. Atlanta: CDC; 2014. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas—2012. Also available from: http://www.cdc.gov/hiv/pdf/surveillance_report_vol_19_no_3.pdf [cited 2015 Apr 24]
-
- Centers for Disease Control and Prevention (US) HIV Surveillance Supplemental Report. No. 4. Vol. 17. Atlanta: CDC; 2012. Estimated HIV incidence in the United States, 2007–2010. Also available from: http://www.cdc.gov/hiv/pdf/statistics_hssr_vol_17_no_4.pdf [cited 2015 Apr 24]
-
- Branson BM, Handsfield HH, Lampe MA, Janssen RS, Taylor AW, Lyss SB, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1–17. - PubMed
-
- Centers for Disease Control and Prevention (US) State HIV testing laws: consent and counseling requirements [cited 2015 Apr 24] Available from: http://www.cdc.gov/hiv/policies/law/states/testing.html.
-
- New York State Department of Health. HIV testing public health law 2014 amendments [cited 2015 Apr 24] Available from: http://www.health.ny.gov/diseases/aids/providers/testing.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical