Self-assembling protein nanoparticles in the design of vaccines
- PMID: 26862374
- PMCID: PMC4706605
- DOI: 10.1016/j.csbj.2015.11.001
Self-assembling protein nanoparticles in the design of vaccines
Abstract
For over 100 years, vaccines have been one of the most effective medical interventions for reducing infectious disease, and are estimated to save millions of lives globally each year. Nevertheless, many diseases are not yet preventable by vaccination. This large unmet medical need demands further research and the development of novel vaccines with high efficacy and safety. Compared to the 19th and early 20th century vaccines that were made of killed, inactivated, or live-attenuated pathogens, modern vaccines containing isolated, highly purified antigenic protein subunits are safer but tend to induce lower levels of protective immunity. One strategy to overcome the latter is to design antigen nanoparticles: assemblies of polypeptides that present multiple copies of subunit antigens in well-ordered arrays with defined orientations that can potentially mimic the repetitiveness, geometry, size, and shape of the natural host-pathogen surface interactions. Such nanoparticles offer a collective strength of multiple binding sites (avidity) and can provide improved antigen stability and immunogenicity. Several exciting advances have emerged lately, including preclinical evidence that this strategy may be applicable for the development of innovative new vaccines, for example, protecting against influenza, human immunodeficiency virus, and respiratory syncytial virus. Here, we provide a concise review of a critical selection of data that demonstrate the potential of this field. In addition, we highlight how the use of self-assembling protein nanoparticles can be effectively combined with the emerging discipline of structural vaccinology for maximum impact in the rational design of vaccine antigens.
Keywords: Ferritin; HBV; HCV; HIV; HPV; Influenza; Lumazine synthase; Malaria; Structural biology; Virus-like particle (VLP).
Figures
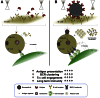
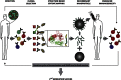
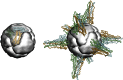
References
-
- Ehreth J. The global value of vaccination. Vaccine. 2003;21:596–600. - PubMed
-
- Klontz K.C., Singh N. Treatment of drug-resistant Shigella infections. Expert Rev Anti Infect Ther. 2015;13:69–80. - PubMed
-
- Takahashi W.N., Ishii M. An abnormal protein associated with tobacco mosaic virus infection. Nature. 1952;169:419–420. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
