TLR4 and TLR7/8 Adjuvant Combinations Generate Different Vaccine Antigen-Specific Immune Outcomes in Minipigs when Administered via the ID or IN Routes
- PMID: 26862758
- PMCID: PMC4749393
- DOI: 10.1371/journal.pone.0148984
TLR4 and TLR7/8 Adjuvant Combinations Generate Different Vaccine Antigen-Specific Immune Outcomes in Minipigs when Administered via the ID or IN Routes
Abstract
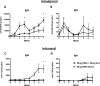
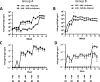
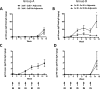
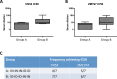
The induction of high levels of systemic and mucosal humoral immunity is a key goal for many prophylactic vaccines. However, adjuvant strategies developed in mice have often performed poorly in the clinic. Due to their closer similarity to humans, minipigs may provide a more accurate picture of adjuvant performance. Based on their complementary signalling pathways, we assessed humoral immune responses to model antigens after co-administration with the toll-like receptor 4 (TLR4) stimulator glucopyranosyl lipid adjuvant (GLA-AF) or the TLR7/8 agonist resiquimod (R848) (alone and in combination) via the intradermal (ID), intranasal (IN) or combined routes in the Gottingen minipig animal model. Surprisingly, we discovered that while GLA-AF additively enhanced the adjuvant effect of R848 when injected ID, it abrogated the adjuvant activity of R848 after IN inoculation. We then performed a route comparison study using a CN54 gp140 HIV Envelope model antigen adjuvanted with R848 + GLA-AF (ID) or R848 alone (IN). Animals receiving priming inoculations via one route were then boosted by the alternate route. Although differences were observed in the priming phase (IN or ID), responses converged upon boosting by the alternative route with no observable impact resultant from the order of administration (ID/IN vs IN/ID). Specific IgG responses were measured at a distal mucosal site (vaginal), although there was no evidence of mucosal linkage as these closely reflected serum antibody levels. These data indicate that the complex in vivo cross-talk between innate pathways are likely tissue specific and cannot be predicted by simple in vitro models.
Conflict of interest statement
Figures



References
-
- Bagchi A, Herrup EA, Warren HS, Trigilio J, Shin HS, Valentine C, et al. MyD88-dependent and MyD88-independent pathways in synergy, priming, and tolerance between TLR agonists. Journal of immunology. 2007;178(2):1164–71. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

