A Chronic Obstructive Pulmonary Disease Susceptibility Gene, FAM13A, Regulates Protein Stability of β-Catenin
- PMID: 26862784
- PMCID: PMC5003213
- DOI: 10.1164/rccm.201505-0999OC
A Chronic Obstructive Pulmonary Disease Susceptibility Gene, FAM13A, Regulates Protein Stability of β-Catenin
Erratum in
-
Erratum: A Chronic Obstructive Pulmonary Disease Susceptibility Gene, FAM13A, Regulates Protein Stability of β-Catenin.Am J Respir Crit Care Med. 2020 Dec 15;202(12):1746. doi: 10.1164/rccm.v202erratum8. Am J Respir Crit Care Med. 2020. PMID: 33320073 Free PMC article. No abstract available.
Abstract
Rationale: A genetic locus within the FAM13A gene has been consistently associated with chronic obstructive pulmonary disease (COPD) in genome-wide association studies. However, the mechanisms by which FAM13A contributes to COPD susceptibility are unknown.
Objectives: To determine the biologic function of FAM13A in human COPD and murine COPD models and discover the molecular mechanism by which FAM13A influences COPD susceptibility.
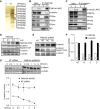
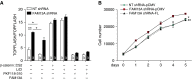
Methods: Fam13a null mice (Fam13a(-/-)) were generated and exposed to cigarette smoke. The lung inflammatory response and airspace size were assessed in Fam13a(-/-) and Fam13a(+/+) littermate control mice. Cellular localization of FAM13A protein and mRNA levels of FAM13A in COPD lungs were assessed using immunofluorescence, Western blotting, and reverse transcriptase-polymerase chain reaction, respectively. Immunoprecipitation followed by mass spectrometry identified cellular proteins that interact with FAM13A to reveal insights on FAM13A's function.
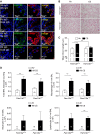
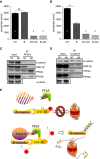
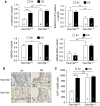
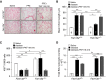
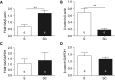
Measurements and main results: In murine and human lungs, FAM13A is expressed in airway and alveolar type II epithelial cells and macrophages. Fam13a null mice (Fam13a(-/-)) were resistant to chronic cigarette smoke-induced emphysema compared with Fam13a(+/+) mice. In vitro, FAM13A interacts with protein phosphatase 2A and recruits protein phosphatase 2A with glycogen synthase kinase 3β and β-catenin, inducing β-catenin degradation. Fam13a(-/-) mice were also resistant to elastase-induced emphysema, and this resistance was reversed by coadministration of a β-catenin inhibitor, suggesting that FAM13A could increase the susceptibility of mice to emphysema development by inhibiting β-catenin signaling. Moreover, human COPD lungs had decreased protein levels of β-catenin and increased protein levels of FAM13A.
Conclusions: We show that FAM13A may influence COPD susceptibility by promoting β-catenin degradation.
Keywords: FAM13A; cell proliferation; emphysema; protein stability; β-catenin.
Figures
Comment in
-
The Translation from Risk Allele to Biological Function in Chronic Obstructive Pulmonary Disease. What's in It for FAM13A?Am J Respir Crit Care Med. 2016 Jul 15;194(2):130-2. doi: 10.1164/rccm.201602-0249ED. Am J Respir Crit Care Med. 2016. PMID: 27420356 No abstract available.
References
-
- Miniño AM. Death in the United States, 2011. NCHS Data Brief. 2013;115:1–8. - PubMed
-
- Miniño AM, Kochanek KD. National Vital Statistics Reports. 2010. Division of Vital Statistics. Deaths: preliminary data for 2008. U.S. Department of Health and Human Services Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System; pp. 1–72. - PubMed
-
- Burrows B, Knudson RJ, Cline MG, Lebowitz MD. Quantitative relationships between cigarette smoking and ventilatory function. Am Rev Respir Dis. 1977;115:195–205. - PubMed
-
- Fletcher C, Peto R, Tinker C, Speizer FE. The natural history of chronic bronchitis and emphysema. Oxford: Oxford University Press; 1976. Factors related to the development of airflow obstruction; pp. 70–105.
-
- McCloskey SC, Patel BD, Hinchliffe SJ, Reid ED, Wareham NJ, Lomas DA. Siblings of patients with severe chronic obstructive pulmonary disease have a significant risk of airflow obstruction. Am J Respir Crit Care Med. 2001;164:1419–1424. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

