Facile rhenium-peptide conjugate synthesis using a one-pot derived Re(CO)3 reagent
- PMID: 26863280
- PMCID: PMC4991029
- DOI: 10.1039/c5dt04694g
Facile rhenium-peptide conjugate synthesis using a one-pot derived Re(CO)3 reagent
Abstract
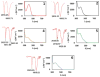
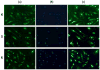
We have synthesized two Re(CO)3-modified lysine complexes (1 and 2), where the metal is attached to the amino acid at the Nε position, via a one-pot Schiff base formation reaction. These compounds can be used in the solid phase synthesis of peptides, and to date we have produced four conjugate systems incorporating neurotensin, bombesin, leutenizing hormone releasing hormone, and a nuclear localization sequence. We observed uptake into human umbilical vascular endothelial cells as well as differential uptake depending on peptide sequence identity, as characterized by fluorescence and rhenium elemental analysis.
Figures


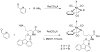

References
-
- Herrick RS, Ziegler CJ, Leeper TC. J Organomet Chem. 2014;751:90–110.
-
- Jaouen G, Vessieres A, Butler IS. Acc Chem Res. 1993:361–369.
-
- Severin K, Bergs R, Beck W. Angew Chemie, Int Ed. 1998;37:1634–1654. - PubMed
-
- Cuingnet E, Sergheraert C, Tartar A, Dautrevaux M. J Organomet Chem. 1980;195:325–329.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

