Analysis of Draize eye irritation testing and its prediction by mining publicly available 2008-2014 REACH data
- PMID: 26863293
- PMCID: PMC5461467
- DOI: 10.14573/altex.1510053
Analysis of Draize eye irritation testing and its prediction by mining publicly available 2008-2014 REACH data
Abstract
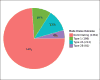
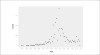
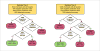
Public data from ECHA online dossiers on 9,801 substances encompassing 326,749 experimental key studies and additional information on classification and labeling were made computable. Eye irritation hazard, for which the rabbit Draize eye test still represents the reference method, was analyzed. Dossiers contained 9,782 Draize eye studies on 3,420 unique substances, indicating frequent retesting of substances. This allowed assessment of the test’s reproducibility based on all substances tested more than once. There was a 10% chance of a non-irritant evaluation after a prior severe-irritant result according to UN GHS classification criteria. The most reproducible outcomes were the results negative (94% reproducible) and severe eye irritant (73% reproducible). To evaluate whether other GHS categorizations predict eye irritation, we built a dataset of 5,629 substances (1,931 “irritant” and 3,698 “non-irritant”). The two best decision trees with up to three other GHS classifications resulted in balanced accuracies of 68% and 73%, i.e., in the rank order of the Draize rabbit eye test itself, but both use inhalation toxicity data (“May cause respiratory irritation”), which is not typically available. Next, a dataset of 929 substances with at least one Draize study was mapped to PubChem to compute chemical similarity using 2D conformational fingerprints and Tanimoto similarity. Using a minimum similarity of 0.7 and simple classification by the closest chemical neighbor resulted in balanced accuracy from 73% over 737 substances to 100% at a threshold of 0.975 over 41 substances. This represents a strong support of read-across and (Q)SAR approaches in this area.
Keywords: animal testing alternatives; chemical safety; dataset; in silico; ocular toxicity.
Conflict of interest statement
The authors have no conflict of interest to state.
Figures




References
-
- Adriaens E, Barroso J, Eskes C, et al. Retrospective analysis of the Draize test for serious eye damage/eye irritation: Importance of understanding the in vivo endpoints under UN GHS/EU CLP for the development and evaluation of in vitro test methods. Arch Toxicol. 2014;88:701–723. http://dx.doi.org/10.1007/s00204-013-1156-8. - DOI - PMC - PubMed
-
- Aha DW, Kibler D, Albert MK. Instance-based learning algorithms. Machine Learning. 1991;6:37–66. http://dx.doi.org/10.1007/BF00153759. - DOI
-
- Andersen FA. Final report on the safety assessment of ascorbyl palmitate, ascorbyl dipalmitate, ascorbyl stearate, erythorbic acid, and sodium erythorbate. Int J Toxicol. 1999;18(Suppl):1–26. http://dx.doi.org/10.1177/109158189901800303. - DOI
-
- Bagley DM, Gardner JR, Holland G, et al. Eye irritation: Updated reference substances data bank. Toxicol In Vitro. 1999;13:505–510. http://dx.doi.org/10.1016/S0887-2333(99)00015-6. - DOI - PubMed
-
- Bastian M, Heymann S, Jacomy M. Gephi: An open source software for exploring and manipulating networks. International AAAI Conference on Weblogs and Social Media (ICWSM) 2009;8:361–362. https://gephi.org/users/publications/
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
