Colon Cancer Tumorigenesis Initiated by the H1047R Mutant PI3K
- PMID: 26863299
- PMCID: PMC4749659
- DOI: 10.1371/journal.pone.0148730
Colon Cancer Tumorigenesis Initiated by the H1047R Mutant PI3K
Abstract
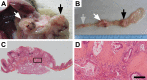
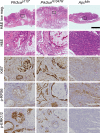
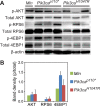
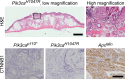
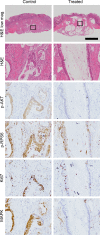
The phosphoinositide 3-kinase (PI3K) signaling pathway is critical for multiple important cellular functions, and is one of the most commonly altered pathways in human cancers. We previously developed a mouse model in which colon cancers were initiated by a dominant active PI3K p110-p85 fusion protein. In that model, well-differentiated mucinous adenocarcinomas developed within the colon and initiated through a non-canonical mechanism that is not dependent on WNT signaling. To assess the potential relevance of PI3K mutations in human cancers, we sought to determine if one of the common mutations in the human disease could also initiate similar colon cancers. Mice were generated expressing the Pik3caH1047R mutation, the analog of one of three human hotspot mutations in this gene. Mice expressing a constitutively active PI3K, as a result of this mutation, develop invasive adenocarcinomas strikingly similar to invasive adenocarcinomas found in human colon cancers. These tumors form without a polypoid intermediary and also lack nuclear CTNNB1 (β-catenin), indicating a non-canonical mechanism of tumor initiation mediated by the PI3K pathway. These cancers are sensitive to dual PI3K/mTOR inhibition indicating dependence on the PI3K pathway. The tumor tissue remaining after treatment demonstrated reduction in cellular proliferation and inhibition of PI3K signaling.
Conflict of interest statement
Figures

References
-
- Goss KH, Groden J. Biology of the adenomatous polyposis coli tumor suppressor. J Clin Oncol. 2000;18:1967–79. - PubMed
-
- Kinzler KW, Vogelstein B. Cancer-susceptibility genes. Gatekeepers and caretakers. Nature. 1997;386:761, 3. - PubMed
-
- Segditsas S, Tomlinson I. Colorectal cancer and genetic alterations in the Wnt pathway. Oncogene. 2006;25:7531–7. - PubMed
-
- Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

