Fluorescence Adherence Inhibition Assay: A Novel Functional Assessment of Blocking Virus Attachment by Vaccine-Induced Antibodies
- PMID: 26863313
- PMCID: PMC4749260
- DOI: 10.1371/journal.pone.0144261
Fluorescence Adherence Inhibition Assay: A Novel Functional Assessment of Blocking Virus Attachment by Vaccine-Induced Antibodies
Abstract
Neutralizing antibodies induced by vaccination or natural infection play a critically important role in protection against the viral diseases. In general, neutralization of the viral infection occurs via two major pathways: pre- and post-attachment modes, the first being the most important for such infections as influenza and polio, the latter being significant for filoviruses. Neutralizing capacity of antibodies is typically evaluated by virus neutralization assays that assess reduction of viral infectivity to the target cells in the presence of functional antibodies. Plaque reduction neutralization test, microneutralization and immunofluorescent assays are often used as gold standard virus neutralization assays. However, these methods are associated with several important prerequisites such as use of live virus requiring safety precautions, tedious evaluation procedure and long assessment time. Hence, there is a need for a robust, inexpensive high throughput functional assay that can be performed rapidly using inactivated virus, without extensive safety precautions. Herein, we report a novel high throughput Fluorescence Adherence Inhibition assay (fADI) using inactivated virus labeled with fluorescent secondary antibodies virus and Vero cells or erythrocytes as targets. It requires only few hours to assess pre-attachment neutralizing capacity of donor sera. fADI assay was tested successfully on donors immunized with polio, yellow fever and influenza vaccines. To further simplify and improve the throughput of the assay, we have developed a mathematical approach for calculating the 50% titers from a single sample dilution, without the need to analyze multi-point titration curves. Assessment of pre- and post-vaccination human sera from subjects immunized with IPOL®, YF-VAX® and 2013-2014 Fluzone® vaccines demonstrated high efficiency of the assay. The results correlated very well with microneutralization assay performed independently by the FDA Center of Biologics Evaluation and Research, with plaque reduction neutralization test performed by Focus Diagnostics, and with hemaglutination inhibition assay performed in-house at Sanofi Pasteur. Taken together, fADI assay appears to be a useful high throughput functional immunoassay for assessment of antibody-related neutralization of the viral infections for which pre-attachment neutralization pathway is predominant, such as polio, influenza, yellow fever and dengue.
Conflict of interest statement
Figures
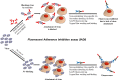

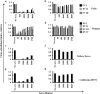
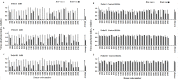

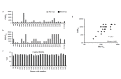
Similar articles
-
The development and validation of a microneutralization assay for the detection and quantification of anti-yellow fever virus antibodies in human serum.Microbiol Spectr. 2025 Apr;13(4):e0334824. doi: 10.1128/spectrum.03348-24. Epub 2025 Mar 4. Microbiol Spectr. 2025. PMID: 40035587 Free PMC article.
-
Mechanism and significance of cell type-dependent neutralization of flaviviruses.J Virol. 2014 Jul;88(13):7210-20. doi: 10.1128/JVI.03690-13. Epub 2014 Apr 16. J Virol. 2014. PMID: 24741083 Free PMC article.
-
Virus Reduction Neutralization Test: A Single-Cell Imaging High-Throughput Virus Neutralization Assay for Dengue.Am J Trop Med Hyg. 2018 Dec;99(6):1430-1439. doi: 10.4269/ajtmh.17-0948. Am J Trop Med Hyg. 2018. PMID: 30350775 Free PMC article.
-
Advances in antiviral vaccine development.Immunol Rev. 2013 Sep;255(1):230-42. doi: 10.1111/imr.12098. Immunol Rev. 2013. PMID: 23947359 Free PMC article. Review.
-
TRIM21-dependent intracellular antibody neutralization of virus infection.Prog Mol Biol Transl Sci. 2015;129:167-87. doi: 10.1016/bs.pmbts.2014.10.006. Epub 2014 Dec 12. Prog Mol Biol Transl Sci. 2015. PMID: 25595804 Review.
Cited by
-
Transchromosomic bovine-derived anti-SARS-CoV-2 polyclonal human antibodies protects hACE2 transgenic hamsters against multiple variants.iScience. 2023 Aug 29;26(10):107764. doi: 10.1016/j.isci.2023.107764. eCollection 2023 Oct 20. iScience. 2023. PMID: 37736038 Free PMC article.
References
-
- Guidelines for Plaque-reduction neutralization assay testing of human antibodies to dengue viruses World Health Organization; (2007). - PubMed
-
- Roberson S (1993) The immunological basis for immunization/module 8: Yellow Fever World Health Organization
-
- Manual for the virological investigation of polio World Health Organization; (1997).
-
- Manual for the laboratory diagnosis and virological surveillance of influenza World Health Organization; (2011).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

