HIF-1α Promotes Glutamine-Mediated Redox Homeostasis and Glycogen-Dependent Bioenergetics to Support Postimplantation Bone Cell Survival
- PMID: 26863487
- PMCID: PMC7611069
- DOI: 10.1016/j.cmet.2016.01.002
HIF-1α Promotes Glutamine-Mediated Redox Homeostasis and Glycogen-Dependent Bioenergetics to Support Postimplantation Bone Cell Survival
Abstract
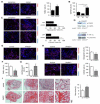
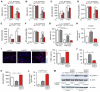
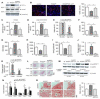
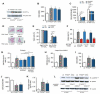
Cell-based therapy is a promising strategy in regenerative medicine, but the poor survival rate of the implanted cells remains a major challenge and limits clinical translation. We preconditioned periosteal cells to the hypoxic and ischemic environment of the bone defect site by deleting prolyl hydroxylase domain-containing protein 2 (PHD2), resulting in hypoxia-inducible factor 1 alpha (HIF-1α) stabilization. This strategy increased postimplantation cell survival and improved bone regeneration. The enhanced cell viability was angiogenesis independent but relied on combined changes in glutamine and glycogen metabolism. HIF-1α stabilization stimulated glutaminase-mediated glutathione synthesis, maintaining redox homeostasis at baseline and during oxidative or nutrient stress. Simultaneously, HIF-1α signaling increased glycogen storage, preventing an energy deficit during nutrient or oxygen deprivation. Pharmacological inhibition of PHD2 recapitulated the adaptations in glutamine and glycogen metabolism and, consequently, the beneficial effects on cell survival. Thus, targeting cellular metabolism is an appealing strategy for bone regeneration and cell-based therapy in general.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Adequate hypoxia inducible factor 1α signaling is indispensable for bone regeneration.Bone. 2016 Jun;87:176-86. doi: 10.1016/j.bone.2016.03.014. Epub 2016 Apr 4. Bone. 2016. PMID: 27058876
-
HIF-1α Stabilization Boosts Pulp Regeneration by Modulating Cell Metabolism.J Dent Res. 2022 Sep;101(10):1214-1226. doi: 10.1177/00220345221091528. Epub 2022 Jul 7. J Dent Res. 2022. PMID: 35798352
-
Oxidative Dimerization of PHD2 is Responsible for its Inactivation and Contributes to Metabolic Reprogramming via HIF-1α Activation.Sci Rep. 2016 Jan 7;6:18928. doi: 10.1038/srep18928. Sci Rep. 2016. PMID: 26740011 Free PMC article.
-
Hypoxia Signaling in the Skeleton: Implications for Bone Health.Curr Osteoporos Rep. 2019 Feb;17(1):26-35. doi: 10.1007/s11914-019-00500-6. Curr Osteoporos Rep. 2019. PMID: 30725321 Free PMC article. Review.
-
Glutaminases regulate glutathione and oxidative stress in cancer.Arch Toxicol. 2020 Aug;94(8):2603-2623. doi: 10.1007/s00204-020-02838-8. Epub 2020 Jul 18. Arch Toxicol. 2020. PMID: 32681190 Review.
Cited by
-
Hypoxia Pathway in Osteoporosis: Laboratory Data for Clinical Prospects.Int J Environ Res Public Health. 2023 Feb 10;20(4):3129. doi: 10.3390/ijerph20043129. Int J Environ Res Public Health. 2023. PMID: 36833823 Free PMC article. Review.
-
The Interaction Between Intracellular Energy Metabolism and Signaling Pathways During Osteogenesis.Front Mol Biosci. 2022 Jan 28;8:807487. doi: 10.3389/fmolb.2021.807487. eCollection 2021. Front Mol Biosci. 2022. PMID: 35155568 Free PMC article. Review.
-
Oxidative Stress in Cancer.Cancer Cell. 2020 Aug 10;38(2):167-197. doi: 10.1016/j.ccell.2020.06.001. Epub 2020 Jul 9. Cancer Cell. 2020. PMID: 32649885 Free PMC article. Review.
-
Beyond the tumor: Enhancing pancreatic cancer therapy through glutamine metabolism and innovative drug delivery.J Cell Commun Signal. 2025 Jul 9;19(3):e70033. doi: 10.1002/ccs3.70033. eCollection 2025 Sep. J Cell Commun Signal. 2025. PMID: 40641572 Free PMC article. Review.
-
Activation of the PGC-1α-mediated mitochondrial glutamine metabolism pathway attenuates female offspring osteoarthritis induced by prenatal excessive prednisone.Sci China Life Sci. 2024 Nov;67(11):2382-2397. doi: 10.1007/s11427-023-2593-4. Epub 2024 Aug 21. Sci China Life Sci. 2024. PMID: 39180608
References
-
- Aragones J, Fraisl P, Baes M, Carmeliet P. Oxygen sensors at the crossroad of metabolism. Cell Metab. 2009;9:11–22. - PubMed
-
- Curcio E, Salerno S, Barbieri G, De Bartolo L, Drioli E, Bader A. Mass transfer and metabolic reactions in hepatocyte spheroids cultured in rotating wall gas-permeable membrane system. Biomaterials. 2007;28:5487–5497. - PubMed
-
- DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

