Classification of large circulating tumor cells isolated with ultra-high throughput microfluidic Vortex technology
- PMID: 26863573
- PMCID: PMC4914319
- DOI: 10.18632/oncotarget.7220
Classification of large circulating tumor cells isolated with ultra-high throughput microfluidic Vortex technology
Abstract
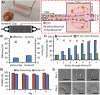
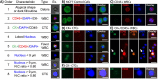
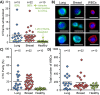
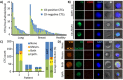
Circulating tumor cells (CTCs) are emerging as rare but clinically significant non-invasive cellular biomarkers for cancer patient prognosis, treatment selection, and treatment monitoring. Current CTC isolation approaches, such as immunoaffinity, filtration, or size-based techniques, are often limited by throughput, purity, large output volumes, or inability to obtain viable cells for downstream analysis. For all technologies, traditional immunofluorescent staining alone has been employed to distinguish and confirm the presence of isolated CTCs among contaminating blood cells, although cells isolated by size may express vastly different phenotypes. Consequently, CTC definitions have been non-trivial, researcher-dependent, and evolving. Here we describe a complete set of objective criteria, leveraging well-established cytomorphological features of malignancy, by which we identify large CTCs. We apply the criteria to CTCs enriched from stage IV lung and breast cancer patient blood samples using the High Throughput Vortex Chip (Vortex HT), an improved microfluidic technology for the label-free, size-based enrichment and concentration of rare cells. We achieve improved capture efficiency (up to 83%), high speed of processing (8 mL/min of 10x diluted blood, or 800 μL/min of whole blood), and high purity (avg. background of 28.8±23.6 white blood cells per mL of whole blood). We show markedly improved performance of CTC capture (84% positive test rate) in comparison to previous Vortex designs and the current FDA-approved gold standard CellSearch assay. The results demonstrate the ability to quickly collect viable and pure populations of abnormal large circulating cells unbiased by molecular characteristics, which helps uncover further heterogeneity in these cells.
Keywords: Vortex; circulating tumor cells; immunofluorescent staining; rare cell enrichment; size based cell isolation.
Conflict of interest statement
D.D. and S.S.J. report grants from Vortex Biosciences. Some of the authors (D.D., E.S., C.R., J.C.) and the Regents of the University of California have financial interests in Vortex Biosciences. D.D. and E.S. is an inventor and D.D. and UCLA receive royalties on intellectual property described herein.
Figures

References
-
- Cristofanilli M, Hayes DF, Budd GT, Ellis MJ, Stopeck A, Reuben JM, Doyle GV, Matera J, Allard WJ, Miller MC, Fritsche HA, Hortobagyi GN, Terstappen LWMM. Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. Journal of Clinical Oncology. 2005;23:1420–30. - PubMed
-
- Pierga J-Y, Bidard F-C, Mathiot C, Brain E, Delaloge S, Giachetti S, de Cremoux P, Salmon R, Vincent-Salomon A, Marty M. Circulating tumor cell detection predicts early metastatic relapse after neoadjuvant chemotherapy in large operable and locally advanced breast cancer in a phase II randomized trial. Clinical Cancer Research. 2008;14:7004–10. - PubMed
-
- Thege FI, Lannin TB, Saha TN, Tsai S, Kochman ML, Hollingsworth MA, Rhim AD, Kirby BJ. Microfluidic immunocapture of circulating pancreatic cells using parallel EpCAM and MUC1 capture: characterization, optimization and downstream analysis. Lab on a Chip. 2014;14:1775–84. - PubMed
-
- Riethdorf S, Fritsche H, Müller V, Rau T, Schindlbeck C, Rack B, Janni W, Coith C, Beck K, Jänicke F, Jackson S, Gornet T, Cristofanilli M, et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clinical Cancer Research. 2007;13:920–8. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

