Randomized Vehicle-Controlled Study of Short Drug Incubation Aminolevulinic Acid Photodynamic Therapy for Actinic Keratoses of the Face or Scalp
- PMID: 26863596
- PMCID: PMC5414777
- DOI: 10.1097/DSS.0000000000000630
Randomized Vehicle-Controlled Study of Short Drug Incubation Aminolevulinic Acid Photodynamic Therapy for Actinic Keratoses of the Face or Scalp
Abstract
Background: Aminolevulinic acid photodynamic therapy (ALA-PDT) can be effective and well tolerated when applied over a broad area and for short drug incubation times.
Objective: To evaluate the effect of short-incubation time and application method on the safety and efficacy of ALA-PDT versus vehicle (VEH-PDT) in the treatment of actinic keratoses (AKs) of the face or scalp.
Methods: Aminolevulinic acid or VEH was applied to face or scalp as a broad area application for 1, 2, or 3 hours or as a spot application for 2 hours before blue light activation. An identical treatment was repeated at Week 8 if any AK lesions remained.
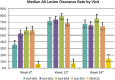
Results: Median AK clearance rate for ALA-treated subjects ranged from 68% to 79% at Week 12, compared with 7% of the VEH-treated group (p < .0001). Complete clearance rate for ALA-treated subjects ranged from 17% (8/46) to 30% (14/47) at Week 12, compared with 2% (1/46) of the VEH-treated group (p = .0041). The safety profile seen in this study is consistent with previously reported side effects of the therapy.
Conclusion: Short-incubation ALA-PDT was found to be superior to VEH-PDT for AK lesion clearance. A second treatment improves efficacy.
Conflict of interest statement
Anna Houlihan and Stuart Marcus of the PDT-AK Investigational Group are both employed by DUSA Pharmaceuticals, Inc.
Figures
References
-
- Criscione VD, Weinstock MA, Naylor MF, Luque C, et al. Actinic keratoses: natural history and risk of malignant transformation in the veterans affairs topical tretinoin chemoprevention trial. Cancer 2009;116:2523–30. - PubMed
-
- Stahl PL, Stranneheim H, Asplund A, Berglund L, et al. Sun-induced nonsynonymous p53 mutations are extensively accumulated and tolerated in normal appearing human skin. J Invest Dermatol 2011;131:504–8. - PubMed
-
- ALA® Kerastick® (aminolevulinic acid HCl) for topical solution, 20% [package insert]. Wilmington, MA: DUSA Pharmaceuticals; 2010.
-
- Piacquadio DJ, Chen DM, Farber HF, Fowler JF, et al. Photodynamic therapy with aminolevulinic acid topical solution and visible blue light in the treatment of multiple actinic keratoses of the face and scalp: investigator-blinded, phase 3, multicenter trials. Arch Dermatol 2004;140:41–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

