Regression/eradication of gliomas in mice by a systemically-deliverable ATF5 dominant-negative peptide
- PMID: 26863637
- PMCID: PMC4914317
- DOI: 10.18632/oncotarget.7212
Regression/eradication of gliomas in mice by a systemically-deliverable ATF5 dominant-negative peptide
Abstract
Malignant gliomas have poor prognosis and urgently require new therapies. Activating Transcription Factor 5 (ATF5) is highly expressed in gliomas, and interference with its expression/function precipitates targeted glioma cell apoptosis in vitro and in vivo. We designed a novel deliverable truncated-dominant-negative (d/n) form of ATF5 fused to a cell-penetrating domain (Pen-d/n-ATF5-RP) that can be intraperitoneally/subcutaneously administered to mice harboring malignant gliomas generated; (1) by PDGF-B/sh-p53 retroviral transformation of endogenous neural progenitor cells; and (2) by human U87-MG xenografts. In vitro Pen-d/n-ATF5-RP entered into glioma cells and triggered massive apoptosis. In vivo, subcutaneously-administered Pen-d/n-ATF5-RP passed the blood brain barrier, entered normal brain and tumor cells, and then caused rapid selective tumor cell death. MRI verified elimination of retrovirus-induced gliomas within 8-21 days. Histopathology revealed growth-suppression of intracerebral human U87-MG cells xenografts. For endogenous PDGF-B gliomas, there was no recurrence or mortality at 6-12 months versus 66% mortality in controls at 6 months. Necropsy and liver-kidney blood enzyme analysis revealed no adverse effects on brain or other tissues. Our findings thus identify Pen-d/n-ATF5-RP as a potential therapy for malignant gliomas.
Keywords: ATF5; apoptosis; brain cancer; cell penetrating peptide; d/n- ATF5.
Conflict of interest statement
Columbia University, on behalf of inventors Drs Angelastro and Greene, has been awarded United States patents US 07888326 “Methods for promoting apoptosis and treating tumor cells by inhibiting the expression or function of the transcription factor ATF5” and US 08158420 “Methods for inhibiting the differentiation of proliferative telencephalic cells
Figures


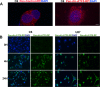
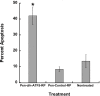




References
-
- Anton K, Baehring JM, Mayer T. Glioblastoma multiforme: overview of current treatment and future perspectives. Hematol Oncol Clin North Am. 2012;26:825–853. - PubMed
-
- Tanaka S, Louis DN, Curry WT, Batchelor TT, Dietrich J. Diagnostic and therapeutic avenues for glioblastoma: no longer a dead end? Nat Rev Clin Oncol. 2013;10:14–26. - PubMed
-
- Angelastro JM, Canoll PD, Kuo J, Weicker M, Costa A, Bruce JN, Greene LA. Selective destruction of glioblastoma cells by interference with the activity or expression of ATF5. Oncogene. 2006;25:907–916. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

