Nonphotochemical Chlorophyll Fluorescence Quenching: Mechanism and Effectiveness in Protecting Plants from Photodamage
- PMID: 26864015
- PMCID: PMC4825125
- DOI: 10.1104/pp.15.01935
Nonphotochemical Chlorophyll Fluorescence Quenching: Mechanism and Effectiveness in Protecting Plants from Photodamage
Abstract
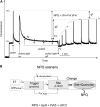
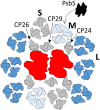
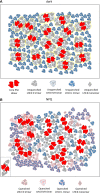
We review the mechanism underlying nonphotochemical chlorophyll fluorescence quenching (NPQ) and its role in protecting plants against photoinhibition. This review includes an introduction to this phenomenon, a brief history of major milestones in our understanding of NPQ, definitions, and a discussion of quantitative measurements of NPQ We discuss the current knowledge and unknown aspects in the NPQ scenario, including the following: ΔpH, the proton gradient (trigger); light-harvesting complex II (LHCII), PSII light harvesting antenna (site); and changes in the antenna induced by ΔpH (change), which lead to the creation of the quencher We conclude that the minimum requirements for NPQ in vivo are ΔpH, LHCII complexes, and the PsbS protein. We highlight the most important unknown in the NPQ scenario, the mechanism by which PsbS acts upon the LHCII antenna. Finally, we describe a novel, emerging technology for assessing the photoprotective "power" of NPQ and the important findings obtained through this technology.
© 2016 American Society of Plant Biologists. All Rights Reserved.
Figures

References
-
- Ahn TK, Avenson TJ, Ballottari M, Cheng YC, Niyogi KK, Bassi R, Fleming GR (2008) Architecture of a charge-transfer state regulating light harvesting in a plant antenna protein. Science 320: 794–797 - PubMed
-
- Åkerlund HE, Andersson B, Persson A, Albertsson PA (1979) Isoelectric points of spinach thylakoid membrane surfaces as determined by cross partition. Biochim Biophys Acta 552: 238–246 - PubMed
-
- Anderson JM, Chow WS, Goodchild DJ (1988) Thylakoid membrane organisation in sun/shade acclimation. Aust J Plant Physiol 15: 11–26
-
- Andrews JR, Fryer MJ, Baker NR (1995) Consequences of LHCII deficiency for photosynthetic regulation in chlorina mutants of barley. Photosynth Res 44: 81–91 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

