The ALSFRS as an outcome measure in therapeutic trials and its relationship to symptom onset
- PMID: 26864085
- PMCID: PMC4950444
- DOI: 10.3109/21678421.2016.1140786
The ALSFRS as an outcome measure in therapeutic trials and its relationship to symptom onset
Abstract
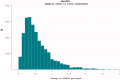
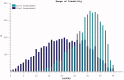
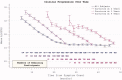
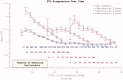
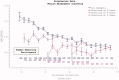
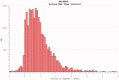
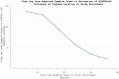
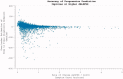
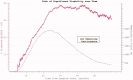
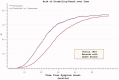
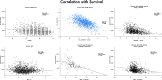
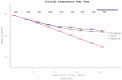
The reduction in ALS Functional Rating Score (ALSFRS) from reported symptom onset to diagnosis is used to estimate rate of disease progression. ALSFRS decline may be non-linear or distorted by drop-outs in therapeutic trials, reducing the reliability of change in slope as an outcome measure. The PRO-ACT database uniquely allows such measures to be explored using historical data from negative therapeutic trials. The decline of functional scores was analysed in 18 pooled trials, comparing rates of decline based on symptom onset with rates calculated between interval assessments. Strategies to mitigate the effects of trial drop-out were considered. Results showed that progression rate calculated by symptom onset underestimated the subsequent rate of disability accumulation, although it predicted survival more accurately than four-month interval estimates of δALSFRS or δFVC. Individual ALSFRS and FVC progression within a typical trial duration were linear. No simple solution to correct for trial drop-out was identified, but imputation using δALSFRS appeared least disruptive. In conclusion, there is a trade-off between the drive to recruit trial participants soon after symptom onset, and reduced reliability of the ALSFRS-derived progression rate at enrolment. The need for objective markers of disease activity as an alternative to survival-based end-points is clear and pressing.
Keywords: Motor neuron disease; clinical trial; modelling; prognosis; survival.
Figures
References
-
- Jablecki CK, Berry C, Leach J. Survival prediction in amyotrophic lateral sclerosis. Muscle Nerve. 1989;12:833–41. - PubMed
-
- Kihira T, Yoshida S, Okamoto K, Kazimoto Y, Ookawa M, Hama K. Survival rate of patients with amyotrophic lateral sclerosis in Wakayama Prefecture, Japan, 1966 to 2005. J Neurol Sci. 2008;268:95–101. - PubMed
-
- Pupillo E, Messina P, Logroscino G, Beghi E. Long-term survival in amyotrophic lateral sclerosis: a population based study. Ann Neurol. 2014;75:287–97. - PubMed
-
- Kaufmann P, Levy G, Thompson JLP, Delbene ML, Battista V, Gordon PH. The ALSFRS-R predicts survival time in an ALS clinic population. Neurology. 2005;64:38–43. - PubMed
MeSH terms
Grants and funding
- MR/L501529/1/MRC_/Medical Research Council/United Kingdom
- MR/K000780/1/MRC_/Medical Research Council/United Kingdom
- TURNER/JAN13/944-795/MNDA_/Motor Neurone Disease Association/United Kingdom
- ALCHALABI-TALBOT/APR14/926-794/MNDA_/Motor Neurone Disease Association/United Kingdom
- MR/K01014X/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
