Regulation of germinal center B-cell differentiation
- PMID: 26864101
- PMCID: PMC4755139
- DOI: 10.1111/imr.12396
Regulation of germinal center B-cell differentiation
Erratum in
-
Corrigendum.Immunol Rev. 2016 Jul;272(1):202. doi: 10.1111/imr.12442. Immunol Rev. 2016. PMID: 27319352 Free PMC article. No abstract available.
Abstract
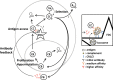
Germinal centers (GC) are the main sites where antigen-activated B-cell clones expand and undergo immunoglobulin gene hypermutation and selection. Iterations of this process will lead to affinity maturation, replicating Darwinian evolution on the cellular level. GC B-cell selection can lead to four different outcomes: further expansion and evolution, apoptosis (non-selection), or output from the GC with differentiation into memory B cells or plasma cells. T-helper cells in GC have been shown to have a central role in regulating B-cell selection by sensing the density of major histocompatibility complex (MHC):peptide antigen complexes. Antigen is provided on follicular dendritic cells in the form of immune complex. Antibody on these immune complexes regulates antigen accessibility by shielding antigen from B-cell receptor access. Replacement of antibody on immune complexes by antibody generated from GC-derived plasma cell output will gradually reduce the availability of antigen. This antibody feedback can lead to a situation where a slow rise in selection stringency caused by a changing environment leads to directional evolution toward higher affinity antibody.
Keywords: B-cell selection; Tfh cells; affinity maturation; cytokines; germinal center; immune complex.
© 2016 The Authors. Immunological Reviews Published by John Wiley & Sons Ltd.
Figures

References
-
- Tarlinton DM. Evolution in miniature: selection, survival and distribution of antigen reactive cells in the germinal centre. Immunol Cell Biol 2008;86:133–138. - PubMed
-
- Regner M. Cross‐reactivity in T‐cell antigen recognition. Immunol Cell Biol 2001;79:91–100. - PubMed
-
- Dal Porto JM, Haberman AM, Shlomchik MJ, Kelsoe G. Antigen drives very low affinity B cells to become plasmacytes and enter germinal centers. J Immunol 1998;161:5373–5381. - PubMed
-
- Shih TA, Meffre E, Roederer M, Nussenzweig MC. Role of BCR affinity in T cell dependent antibody responses in vivo. Nat Immunol 2002;3:570–575. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

