Engineered hapten-binding antibody derivatives for modulation of pharmacokinetic properties of small molecules and targeted payload delivery
- PMID: 26864111
- PMCID: PMC4755198
- DOI: 10.1111/imr.12386
Engineered hapten-binding antibody derivatives for modulation of pharmacokinetic properties of small molecules and targeted payload delivery
Abstract
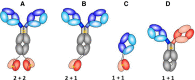
Hapten-binding antibodies have for more than 50 years played a pivotal role in immunology, paving the way to antibody generation (as haptens are very important and robust immunogens), to antibody characterization (as the first structures generated more than 40 years ago were those of hapten binders), and enabled and expanded antibody engineering technologies. The latter field of engineered antibodies evolved over many years and many steps resulting in recombinant humanized or human-derived antibody derivatives in multiple formats. Today, hapten-binding antibodies are applied not only as reagents and tools (where they still play an important part) but evolved also to engineered targeting and pretargeting vehicles for disease diagnosis and therapy. Here we describe recent applications of hapten-binding antibodies and of engineered mono- and bispecific hapten-binding antibody derivatives. We have designed and applied these molecules for the modulation of the pharmacokinetic properties of small compounds or peptides. They are also integrated as additional binding entities into bispecific antibody formats. Here they serve as non-covalent or covalent coupling modules to haptenylated compounds, to enable targeted payload delivery to disease tissues or cells.
Keywords: biotin; digoxigenin; protein engineering; protein structure; targeted therapy.
© 2016 Authors. Immunological Reviews Published by John Wiley & Sons Ltd.
Figures



Similar articles
-
Hapten-directed spontaneous disulfide shuffling: a universal technology for site-directed covalent coupling of payloads to antibodies.FASEB J. 2015 May;29(5):1763-79. doi: 10.1096/fj.14-263665. Epub 2015 Feb 10. FASEB J. 2015. PMID: 25670234 Free PMC article.
-
Hapten-Binding Bispecific Antibodies for the Targeted Delivery of SiRNA and SiRNA-Containing Nanoparticles.Methods Mol Biol. 2016;1364:219-34. doi: 10.1007/978-1-4939-3112-5_18. Methods Mol Biol. 2016. PMID: 26472454
-
Generation of dual-variable-domain immunoglobulin molecules for dual-specific targeting.Methods Enzymol. 2012;502:25-41. doi: 10.1016/B978-0-12-416039-2.00002-1. Methods Enzymol. 2012. PMID: 22208980
-
Current strategies in antibody engineering: Fc engineering and pH-dependent antigen binding, bispecific antibodies and antibody drug conjugates.Biotechnol J. 2012 Dec;7(12):1444-50. doi: 10.1002/biot.201200250. Epub 2012 Nov 1. Biotechnol J. 2012. PMID: 23125076 Review.
-
[Bispecific antibodies: what future?].Med Sci (Paris). 2009 Dec;25(12):1155-8. doi: 10.1051/medsci/200925121155. Med Sci (Paris). 2009. PMID: 20035697 Review. French.
Cited by
-
Nasal Nanovaccines for SARS-CoV-2 to Address COVID-19.Vaccines (Basel). 2022 Mar 8;10(3):405. doi: 10.3390/vaccines10030405. Vaccines (Basel). 2022. PMID: 35335037 Free PMC article. Review.
-
Immunosensor of Nitrofuran Antibiotics and Their Metabolites in Animal-Derived Foods: A Review.Front Chem. 2022 Jun 1;10:813666. doi: 10.3389/fchem.2022.813666. eCollection 2022. Front Chem. 2022. PMID: 35721001 Free PMC article. Review.
-
Bispecific Antibodies as a Development Platform for New Concepts and Treatment Strategies.Int J Mol Sci. 2016 Dec 28;18(1):48. doi: 10.3390/ijms18010048. Int J Mol Sci. 2016. PMID: 28036020 Free PMC article. Review.
-
MicroRNA-Directed Neuronal Reprogramming as a Therapeutic Strategy for Neurological Diseases.Mol Neurobiol. 2018 May;55(5):4428-4436. doi: 10.1007/s12035-017-0671-7. Epub 2017 Jun 29. Mol Neurobiol. 2018. PMID: 28664454 Review.
-
Structural basis for chemically-induced homodimerization of a single domain antibody.Sci Rep. 2019 Feb 12;9(1):1840. doi: 10.1038/s41598-019-38752-y. Sci Rep. 2019. PMID: 30755682 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

