Green fluorescent protein as a scaffold for high efficiency production of functional bacteriotoxic proteins in Escherichia coli
- PMID: 26864123
- PMCID: PMC4749965
- DOI: 10.1038/srep20661
Green fluorescent protein as a scaffold for high efficiency production of functional bacteriotoxic proteins in Escherichia coli
Abstract
The availability of simple, robust, and cost-effective methods for the large-scale production of bacteriotoxic peptides such as antimicrobial peptides (AMPs) is essential for basic and pharmaceutical research. However, the production of bacteriotoxic proteins has been difficult due to a high degree of toxicity in bacteria and proteolytic degradation. In this study, we inserted AMPs into the Green fluorescent protein (GFP) in a loop region and expressed them as insoluble proteins in high yield, circumventing the inherent toxicity of AMP production in Escherichia coli. The AMPs inserted were released by cyanogen bromide and purified by chromatography. We showed that highly potent AMPs such as Protegrin-1, PMAP-36, Buforin-2, and Bactridin-1 are produced in high yields and produced AMPs showed similar activities compared to chemically synthesized AMPs. We increased the yield more than two-fold by inserting three copies of Protegrin-1 in the GFP scaffold. The immunogold electron micrographs showed that the expressed Protegrin-1 in the GFP scaffold forms large and small size aggregates in the core region of the inclusion body and become entirely nonfunctional, therefore not influencing the proliferation of E. coli. Our novel method will be applicable for diverse bacteriotoxic peptides which can be exploited in biomedical and pharmaceutical researches.
Conflict of interest statement
The authors declare competing financial interests. The r5M-172-AMP-173 technologies are the subject of domestic and foreign patent applications by Konkuk University.
Figures

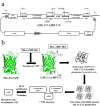

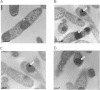
References
-
- Achmuller C. et al. Npro fusion technology to produce proteins with authentic N termini in E. coli. Nat. Meth. 4, 1037–1043 (2007). - PubMed
-
- Lee J. H. et al. High-level expression of antimicrobial peptide mediated by a fusion partner reinforcing formation of inclusion bodies. Biochem. Biophs. Res. Commun. 277, 575–580 (2000). - PubMed
-
- Vidovic V., Prongidi-Fix L., Bechinger B. & Werten S. Production and isotope labeling of antimicrobial peptides in Escherichia coli by means of a novel fusion partner that enables high-yield insoluble expression and fast purification. J. Pept. Sci. 15, 278–284 (2009). - PubMed
-
- Hara S. & Yamakawa M. Production in Escherichia coli of moricin, a novel type antibacterial peptide from the Silkworm,Bombyx mori. Biochem. Biophs. Res. Commun. 220, 664–669 (1996). - PubMed
-
- Piers K. L., Brown M. H. & Hancock R. E. W. Recombinant DNA procedures for producing small antimicrobial cationic peptides in bacteria. Gene. 134, 7–13 (1993). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

