Genomic characterization of sarcomatoid transformation in clear cell renal cell carcinoma
- PMID: 26864202
- PMCID: PMC4776463
- DOI: 10.1073/pnas.1525735113
Genomic characterization of sarcomatoid transformation in clear cell renal cell carcinoma
Abstract
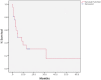
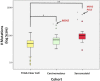
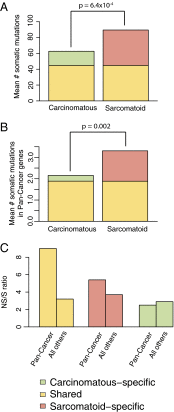
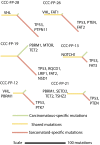
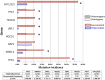
The presence of sarcomatoid features in clear cell renal cell carcinoma (ccRCC) confers a poor prognosis and is of unknown pathogenesis. We performed exome sequencing of matched normal-carcinomatous-sarcomatoid specimens from 21 subjects. Two tumors had hypermutation consistent with mismatch repair deficiency. In the remainder, sarcomatoid and carcinomatous elements shared 42% of somatic single-nucleotide variants (SSNVs). Sarcomatoid elements had a higher overall SSNV burden (mean 90 vs. 63 SSNVs, P = 4.0 × 10(-4)), increased frequency of nonsynonymous SSNVs in Pan-Cancer genes (mean 1.4 vs. 0.26, P = 0.002), and increased frequency of loss of heterozygosity (LOH) across the genome (median 913 vs. 460 Mb in LOH, P < 0.05), with significant recurrent LOH on chromosomes 1p, 9, 10, 14, 17p, 18, and 22. The most frequent SSNVs shared by carcinomatous and sarcomatoid elements were in known ccRCC genes including von Hippel-Lindau tumor suppressor (VHL), polybromo 1 (PBRM1), SET domain containing 2 (SETD2), phosphatase and tensin homolog (PTEN). Most interestingly, sarcomatoid elements acquired biallelic tumor protein p53 (TP53) mutations in 32% of tumors (P = 5.47 × 10(-17)); TP53 mutations were absent in carcinomatous elements in nonhypermutated tumors and rare in previously studied ccRCCs. Mutations in known cancer drivers AT-rich interaction domain 1A (ARID1A) and BRCA1 associated protein 1 (BAP1) were significantly mutated in sarcomatoid elements and were mutually exclusive with TP53 and each other. These findings provide evidence that sarcomatoid elements arise from dedifferentiation of carcinomatous ccRCCs and implicate specific genes in this process. These findings have implications for the treatment of patients with these poor-prognosis cancers.
Keywords: dedifferentiation; kidney cancer; p53; sarcomatoid; transformation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- de Peralta-Venturina M, et al. Sarcomatoid differentiation in renal cell carcinoma: A study of 101 cases. Am J Surg Pathol. 2001;25(3):275–284. - PubMed
-
- Shuch B, et al. Understanding pathologic variants of renal cell carcinoma: Distilling therapeutic opportunities from biologic complexity. Eur Urol. 2015;67(1):85–97. - PubMed
-
- Zhang BY, et al. A novel prognostic model for patients with sarcomatoid renal cell carcinoma. BJU Int. 2015;115(3):405–411. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

