IL-33 mediates reactive eosinophilopoiesis in response to airborne allergen exposure
- PMID: 26864308
- PMCID: PMC5107318
- DOI: 10.1111/all.12861
IL-33 mediates reactive eosinophilopoiesis in response to airborne allergen exposure
Abstract
Background: Exposure to aeroallergens induces eosinophilic airway inflammation in patients with asthma and allergic airway diseases. The circulating number of eosinophils in peripheral blood is relatively small, leading us to hypothesize that bone marrow needs to be engaged quickly to meet the demands of the tissues.
Methods: To investigate the communication between the lungs and bone marrow, we used acute allergen exposure and airway inflammation models in mice. Gene-deficient mice and cytokine reporter mice as well as in vitro cell culture models were used to dissect the mechanisms.
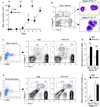
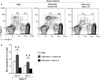
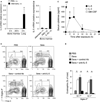
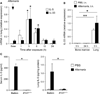
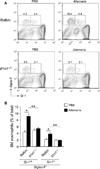
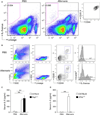
Results: Naïve BALB/c mice produced increased numbers of eosinophil precursors and mature eosinophils in the bone marrow when their airways were exposed to a common fungal allergen, Alternaria alternata. Expression of IL-5 and IL-33 increased rapidly in the lungs, but not in the bone marrow. Sera from allergen-exposed mice promoted eosinophilopoiesis in bone marrow cells from naïve mice, which was blocked by anti-IL-5 antibody. Mice deficient in the IL-33 receptor ST2 (i.e., Il1rl1(-/-) mice) were unable to increase their serum levels of IL-5 and allergen-induced eosinophilopoiesis in the bone marrow after allergen exposure. Finally, group 2 innate lymphoid cells (ILC2s) in the lungs showed robust expression of IL-5 after Alternaria exposure.
Conclusions: These finding suggests that lung IL-33, through innate activation of ILC2s and their production of IL-5, plays a key role in promoting acute reactive eosinophilopoiesis in the bone marrow when naïve animals are exposed to airborne allergens. Therefore, bone marrow eosinophilopoiesis may be affected by atmospheric environmental conditions.
Keywords: IL-33; IL-5; eosinophilopoiesis; eosinophils.
© 2016 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
References
-
- Wenzel SE. Asthma phenotypes: The evolution from clinical to molecular approaches. Nat Med. 2012;18:716–725. - PubMed
-
- Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–174. - PubMed
-
- Sur S, Crotty TB, Kephart GM, Hyma BA, Colby TV, Reed CE, et al. Sudden-onset fatal asthma. A distinct entity with few eosinophils and relatively more neutrophils in the airway submucosa? Am Rev Respir Dis. 1993;148:713–719. - PubMed
-
- Griffiths-Johnson DA, Collins PD, Rossi AG, Jose PJ, Williams TJ. The chemokine, eotaxin, activates guinea-pig eosinophils in vitro and causes their accumulation into the lung in vivo. Biochem Biophys Res Commun. 1993;197:1167–1172. - PubMed
-
- Bochner BS, Schleimer RP. Mast cells, basophils, and eosinophils: distinct but overlapping pathways for recruitment. Immunol Rev. 2001;179:5–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

