Bioelectric signalling via potassium channels: a mechanism for craniofacial dysmorphogenesis in KCNJ2-associated Andersen-Tawil Syndrome
- PMID: 26864374
- PMCID: PMC4908029
- DOI: 10.1113/JP271930
Bioelectric signalling via potassium channels: a mechanism for craniofacial dysmorphogenesis in KCNJ2-associated Andersen-Tawil Syndrome
Abstract
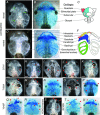
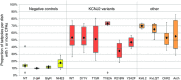
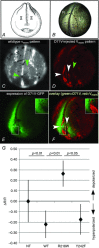
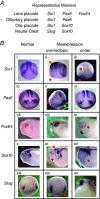
Key points: Xenopus laevis craniofacial development is a good system for the study of Andersen-Tawil Syndrome (ATS)-associated craniofacial anomalies (CFAs) because (1) Kcnj2 is expressed in the nascent face; (2) molecular-genetic and biophysical techniques are available for the study of ion-dependent signalling during craniofacial morphogenesis; (3) as in humans, expression of variant Kcnj2 forms in embryos causes a muscle phenotype; and (4) variant forms of Kcnj2 found in human patients, when injected into frog embryos, cause CFAs in the same cell lineages. Forced expression of WT or variant Kcnj2 changes the normal pattern of Vmem (resting potential) regionalization found in the ectoderm of neurulating embryos, and changes the normal pattern of expression of ten different genetic regulators of craniofacial development, including markers of cranial neural crest and of placodes. Expression of other potassium channels and two different light-activated channels, all of which have an effect on Vmem , causes CFAs like those induced by injection of Kcnj2 variants. In contrast, expression of Slc9A (NHE3), an electroneutral ion channel, and of GlyR, an inactive Cl(-) channel, do not cause CFAs, demonstrating that correct craniofacial development depends on a pattern of bioelectric states, not on ion- or channel-specific signalling. Using optogenetics to control both the location and the timing of ion flux in developing embryos, we show that affecting Vmem of the ectoderm and no other cell layers is sufficient to cause CFAs, but only during early neurula stages. Changes in Vmem induced late in neurulation do not affect craniofacial development. We interpret these data as strong evidence, consistent with our hypothesis, that ATS-associated CFAs are caused by the effect of variant Kcnj2 on the Vmem of ectodermal cells of the developing face. We predict that the critical time is early during neurulation, and the critical cells are the ectodermal cranial neural crest and placode lineages. This points to the potential utility of extant, ion flux-modifying drugs as treatments to prevent CFAs associated with channelopathies such as ATS.
Abstract: Variants in potassium channel KCNJ2 cause Andersen-Tawil Syndrome (ATS); the induced craniofacial anomalies (CFAs) are entirely unexplained. We show that KCNJ2 is expressed in Xenopus and mouse during the earliest stages of craniofacial development. Misexpression in Xenopus of KCNJ2 carrying ATS-associated mutations causes CFAs in the same structures affected in humans, changes the normal pattern of membrane voltage potential regionalization in the developing face and disrupts expression of important craniofacial patterning genes, revealing the endogenous control of craniofacial patterning by bioelectric cell states. By altering cells' resting potentials using other ion translocators, we show that a change in ectodermal voltage, not tied to a specific protein or ion, is sufficient to cause CFAs. By adapting optogenetics for use in non-neural cells in embryos, we show that developmentally patterned K(+) flux is required for correct regionalization of the resting potentials and for establishment of endogenous early gene expression domains in the anterior ectoderm, and that variants in KCNJ2 disrupt this regionalization, leading to the CFAs seen in ATS patients.
© 2016 The Authors. The Journal of Physiology © 2016 The Physiological Society.
Figures





References
-
- Abe M, Maeda T & Wakisaka S (2008). Retinoic acid affects craniofacial patterning by changing Fgf8 expression in the pharyngeal ectoderm. Dev Growth Differ 50, 717–729. - PubMed
-
- Abello G, Khatri S, Radosevic M, Scotting PJ, Giraldez F & Alsina B (2010). Independent regulation of Sox3 and Lmx1b by FGF and BMP signalling influences the neurogenic and non‐neurogenic domains in the chick otic placode. Dev Biol 339, 166–178. - PubMed
-
- Adams DS (2008). A new tool for tissue engineers: ions as regulators of morphogenesis during development and regeneration. Tissue Eng Part A 14, 1461–1468. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

