A simple, step-by-step dissection protocol for the rapid isolation of mouse dorsal root ganglia
- PMID: 26864470
- PMCID: PMC4750296
- DOI: 10.1186/s13104-016-1915-8
A simple, step-by-step dissection protocol for the rapid isolation of mouse dorsal root ganglia
Abstract
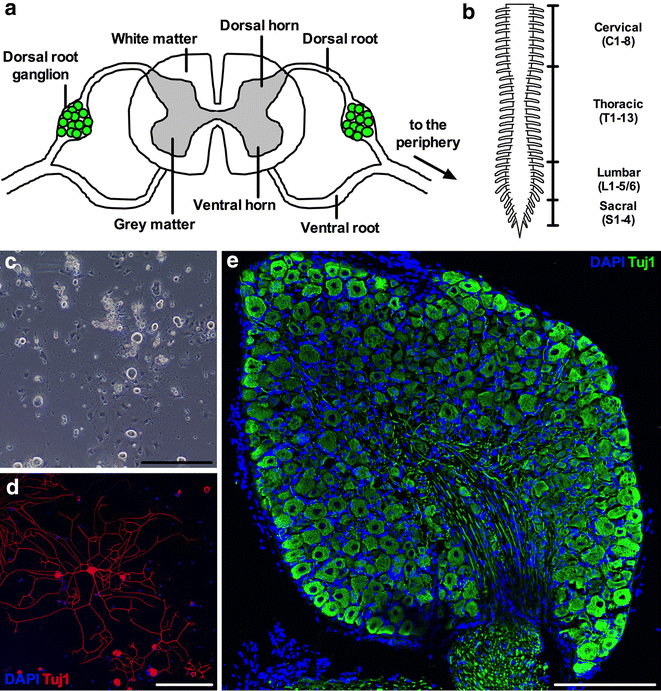
Background: The cell bodies of sensory neurons, which transmit information from the external environment to the spinal cord, can be found at all levels of the spinal column in paired structures called dorsal root ganglia (DRG). Rodent DRG neurons have long been studied in the laboratory to improve understanding of sensory nerve development and function, and have been instrumental in determining mechanisms underlying pain and neurodegeneration in disorders of the peripheral nervous system. Here, we describe a simple, step-by-step protocol for the swift isolation of mouse DRG, which can be enzymatically dissociated to produce fully differentiated primary neuronal cultures, or processed for downstream analyses, such as immunohistochemistry or RNA profiling.
Findings: After dissecting out the spinal column, from the base of the skull to the level of the femurs, it can be cut down the mid-line and the spinal cord and meninges removed, before extracting the DRG and detaching unwanted axons. This protocol allows the easy and rapid isolation of DRG with minimal practice and dissection experience. The process is both faster and less technically challenging than extracting the ganglia from the in situ column after performing a dorsal laminectomy.
Conclusions: This approach reduces the time required to collect DRG, thereby improving efficiency, permitting less opportunity for tissue deterioration, and, ultimately, increasing the chances of generating healthy primary DRG cultures or high quality, reproducible experiments using DRG tissue.
Figures



Similar articles
-
A video protocol for rapid dissection of mouse dorsal root ganglia from defined spinal levels.BMC Res Notes. 2020 Jun 24;13(1):302. doi: 10.1186/s13104-020-05147-6. BMC Res Notes. 2020. PMID: 32580748 Free PMC article.
-
Regeneration of primary sensory axons into the adult rat spinal cord via a peripheral nerve graft bridging the lumbar dorsal roots to the dorsal column.J Neurosci Res. 2002 May 1;68(3):293-304. doi: 10.1002/jnr.10179. J Neurosci Res. 2002. PMID: 12111859
-
Stimulation of the Dorsal Root Ganglion.Prog Neurol Surg. 2015;29:213-24. doi: 10.1159/000434673. Epub 2015 Sep 4. Prog Neurol Surg. 2015. PMID: 26394301 Review.
-
Dorsally derived netrin 1 provides an inhibitory cue and elaborates the 'waiting period' for primary sensory axons in the developing spinal cord.Development. 2006 Apr;133(7):1379-87. doi: 10.1242/dev.02312. Epub 2006 Mar 1. Development. 2006. PMID: 16510500
-
Ultrastructure of dorsal root ganglia.Cell Tissue Res. 2023 Jul;393(1):17-36. doi: 10.1007/s00441-023-03770-w. Epub 2023 Apr 20. Cell Tissue Res. 2023. PMID: 37079097 Free PMC article. Review.
Cited by
-
Semaphorin3A induces nerve regeneration in the adult cornea-a switch from its repulsive role in development.PLoS One. 2018 Jan 25;13(1):e0191962. doi: 10.1371/journal.pone.0191962. eCollection 2018. PLoS One. 2018. PMID: 29370308 Free PMC article.
-
Age-specific and compartment-dependent changes in mitochondrial homeostasis and cytoplasmic viscosity in mouse peripheral neurons.Aging Cell. 2024 Oct;23(10):e14250. doi: 10.1111/acel.14250. Epub 2024 Jun 17. Aging Cell. 2024. PMID: 38881280 Free PMC article.
-
Rapid injection of lumbar dorsal root ganglia under direct vision: Relevant anatomy, protocol, and behaviors.Front Neurol. 2023 Apr 11;14:1138933. doi: 10.3389/fneur.2023.1138933. eCollection 2023. Front Neurol. 2023. PMID: 37114234 Free PMC article.
-
Analysis of the immune response to sciatic nerve injury identifies efferocytosis as a key mechanism of nerve debridement.Elife. 2020 Dec 2;9:e60223. doi: 10.7554/eLife.60223. Elife. 2020. PMID: 33263277 Free PMC article.
-
Triple-Negative Breast Cancer Cells Activate Sensory Neurons via TRPV1 to Drive Nerve Outgrowth and Tumor Progression.bioRxiv [Preprint]. 2025 Jul 26:2025.07.22.666151. doi: 10.1101/2025.07.22.666151. bioRxiv. 2025. PMID: 40777234 Free PMC article. Preprint.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

