Electrical synapses and the development of inhibitory circuits in the thalamus
- PMID: 26864476
- PMCID: PMC4865577
- DOI: 10.1113/JP271880
Electrical synapses and the development of inhibitory circuits in the thalamus
Abstract
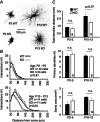
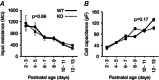
Key points: The thalamus is a structure critical for information processing and transfer to the cortex. Thalamic reticular neurons are inhibitory cells interconnected by electrical synapses, most of which require the gap junction protein connexin36 (Cx36). We investigated whether electrical synapses play a role in the maturation of thalamic networks by studying neurons in mice with and without Cx36. When Cx36 was deleted, inhibitory synapses were more numerous, although both divergent inhibitory connectivity and dendritic complexity were reduced. Surprisingly, we observed non-Cx36-dependent electrical synapses with unusual biophysical properties interconnecting some reticular neurons in mice lacking Cx36. The results of the present study suggest an important role for Cx36-dependent electrical synapses in the development of thalamic circuits.
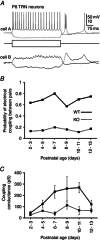
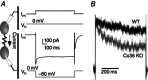
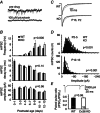
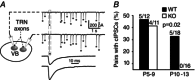
Abstract: Neurons within the mature thalamic reticular nucleus (TRN) powerfully inhibit ventrobasal (VB) thalamic relay neurons via GABAergic synapses. TRN neurons are also coupled to one another by electrical synapses that depend strongly on the gap junction protein connexin36 (Cx36). Electrical synapses in the TRN precede the postnatal development of TRN-to-VB inhibition. We investigated how the deletion of Cx36 affects the maturation of TRN and VB neurons, electrical coupling and GABAergic synapses by studying wild-type (WT) and Cx36 knockout (KO) mice. The incidence and strength of electrical coupling in TRN was sharply reduced, but not abolished, in KO mice. Surprisingly, electrical synapses between Cx36-KO neurons had faster voltage-dependent decay kinetics and conductance asymmetry (rectification) than did electrical synapses between WT neurons. The properties of TRN-mediated inhibition in VB also depended on the Cx36 genotype. Deletion of Cx36 increased the frequency and shifted the amplitude distributions of miniature IPSCs, whereas the paired-pulse ratio of evoked IPSCs was unaffected, suggesting that the absence of Cx36 led to an increase in GABAergic synaptic contacts. VB neurons from Cx36-KO mice also tended to have simpler dendritic trees and fewer divergent inputs from the TRN compared to WT cells. The findings obtained in the present study suggest that proper development of thalamic inhibitory circuitry, neuronal morphology, TRN cell function and electrical coupling requires Cx36. In the absence of Cx36, some TRN neurons express asymmetric electrical coupling mediated by other unidentified connexin subtypes.
© 2016 The Authors. The Journal of Physiology © 2016 The Physiological Society.
Figures



Comment in
-
Developmental functions of electrical synapses.J Physiol. 2016 May 15;594(10):2561-2. doi: 10.1113/JP272361. J Physiol. 2016. PMID: 27173021 Free PMC article. No abstract available.
Similar articles
-
Electrical synapses between inhibitory neurons shape the responses of principal neurons to transient inputs in the thalamus: a modeling study.Sci Rep. 2018 May 17;8(1):7763. doi: 10.1038/s41598-018-25956-x. Sci Rep. 2018. PMID: 29773817 Free PMC article.
-
Electrical and chemical synapses between relay neurons in developing thalamus.J Physiol. 2010 Jul 1;588(Pt 13):2403-15. doi: 10.1113/jphysiol.2010.187096. Epub 2010 May 10. J Physiol. 2010. PMID: 20457735 Free PMC article.
-
Electrical synapses in the thalamic reticular nucleus.J Neurosci. 2002 Feb 1;22(3):1002-9. doi: 10.1523/JNEUROSCI.22-03-01002.2002. J Neurosci. 2002. PMID: 11826128 Free PMC article.
-
Connexon connexions in the thalamocortical system.Prog Brain Res. 2005;149:41-57. doi: 10.1016/S0079-6123(05)49004-4. Prog Brain Res. 2005. PMID: 16226575 Review.
-
Electrical synapses in the mammalian brain.Annu Rev Neurosci. 2004;27:393-418. doi: 10.1146/annurev.neuro.26.041002.131128. Annu Rev Neurosci. 2004. PMID: 15217338 Review.
Cited by
-
Understanding electrical and chemical transmission in the brain.Front Cell Neurosci. 2024 Jun 26;18:1398862. doi: 10.3389/fncel.2024.1398862. eCollection 2024. Front Cell Neurosci. 2024. PMID: 38988663 Free PMC article. Review.
-
Disruption of Cholinergic Retinal Waves Alters Visual Cortex Development and Function.bioRxiv [Preprint]. 2024 Apr 15:2024.04.05.588143. doi: 10.1101/2024.04.05.588143. bioRxiv. 2024. PMID: 38644996 Free PMC article. Preprint.
-
Circuits and components of delta wave regulation.Brain Res Bull. 2022 Oct 1;188:223-232. doi: 10.1016/j.brainresbull.2022.06.006. Epub 2022 Jun 20. Brain Res Bull. 2022. PMID: 35738502 Free PMC article. Review.
-
Physiologic regulation of heart rate and blood pressure involves connexin 36-containing gap junctions.FASEB J. 2017 Sep;31(9):3966-3977. doi: 10.1096/fj.201600919RR. Epub 2017 May 22. FASEB J. 2017. PMID: 28533325 Free PMC article.
-
Synaptic Functions of Hemichannels and Pannexons: A Double-Edged Sword.Front Mol Neurosci. 2018 Dec 4;11:435. doi: 10.3389/fnmol.2018.00435. eCollection 2018. Front Mol Neurosci. 2018. PMID: 30564096 Free PMC article. Review.
References
-
- Agmon A & Connors BW (1991). Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience 41, 365–379. - PubMed
-
- Arumugam H, Liu X, Colombo PJ, Corriveau RA & Belousov AB (2005). NMDA receptors regulate developmental gap junction uncoupling via CREB signaling. Nat Neurosci 8, 1720–1726. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

