Expression of cytokine and apoptosis-related genes in bovine peripheral blood mononuclear cells stimulated with Brucella abortus recombinant proteins
- PMID: 26864657
- PMCID: PMC4750197
- DOI: 10.1186/s13567-016-0311-7
Expression of cytokine and apoptosis-related genes in bovine peripheral blood mononuclear cells stimulated with Brucella abortus recombinant proteins
Abstract
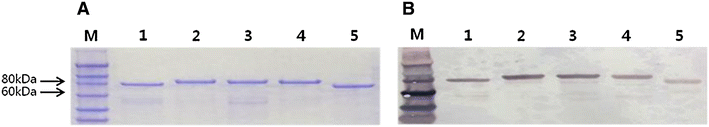
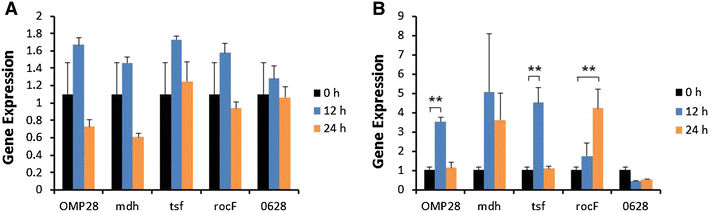
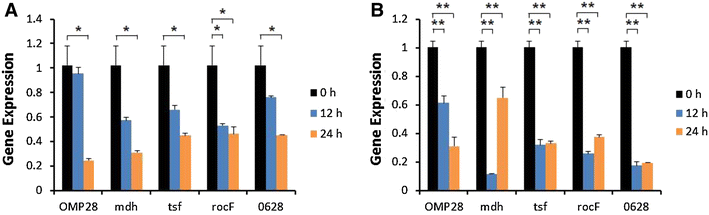
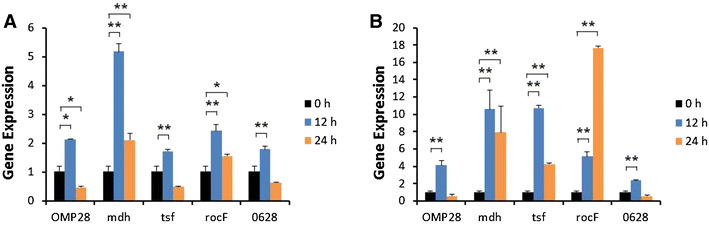
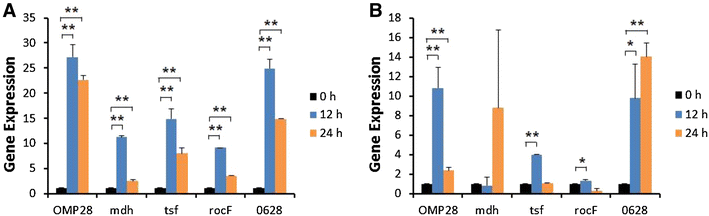
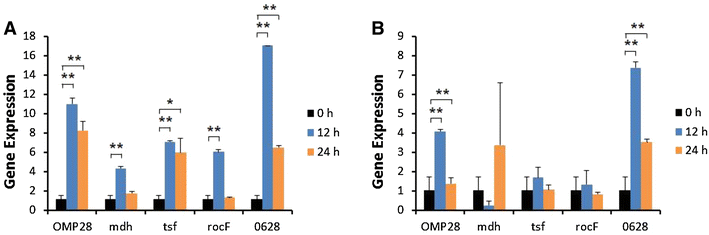
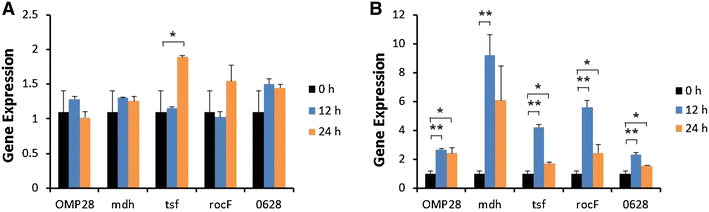
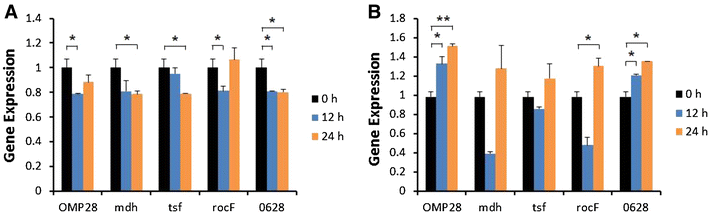
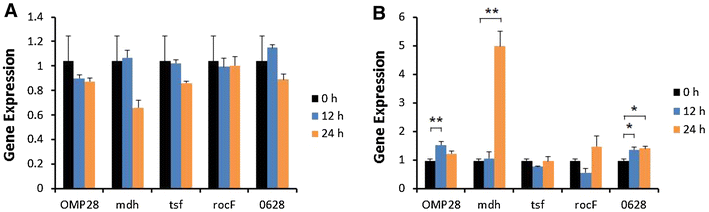
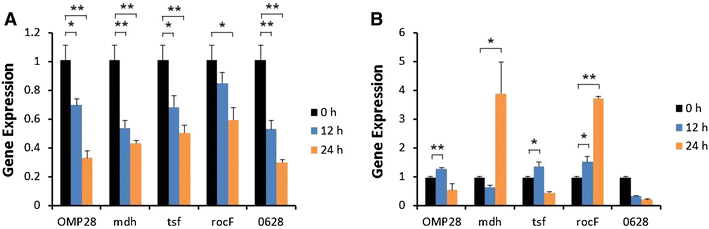
Brucellosis is a clinically and economically important disease. Therefore, eradication programs of the disease have been implemented in several countries. One hurdle in these programs is the detection of infected animals at the early stage. Although the protein antigens as diagnostic antigens have recently received attention, the exact mechanisms at the beginning of immune responses are not yet known. Therefore, genes encoding five B. abortus cellular proteins were cloned and the expressed recombinant proteins were purified. The expression of several cytokine genes (IL-1β, IL-4, IL-6, IL-12p40, IFN-γ, TNF-α, and iNOS) was analyzed in bovine peripheral blood mononuclear cells (bPBMC) after stimulation with the recombinant proteins. Three apoptosis-related genes, Bax, Bcl-2, and TLR4, were also included in the analysis to find out the adverse effects of the proteins to the cells. Each protein induced different patterns of cytokine expression depending on the stimulation time and antigen dose. Expression of IL-6, IL-12p40, and IFN-γ was induced with all of the proteins while IL-1β, IL-4, TNF-α, and iNOS gene expression was not. Expression of apoptosis-related genes was not altered except TLR4. These results suggest that the cellular antigens of B. abortus induce both humoral and cellular immunity via the production of IL-6, IL-12p40, and IFN-γ in bPBMC without exerting any adverse effects on the cells.
Figures
Similar articles
-
Characterization of Brucella abortus infection of bovine monocyte-derived dendritic cells.Vet Immunol Immunopathol. 2012 Oct 15;149(3-4):255-61. doi: 10.1016/j.vetimm.2012.07.006. Epub 2012 Jul 22. Vet Immunol Immunopathol. 2012. PMID: 22884262
-
Recombinant bovine interleukin 2 enhances immunity and protection induced by Brucella abortus vaccines in cattle.Vet Microbiol. 2005 Nov 30;111(1-2):77-87. doi: 10.1016/j.vetmic.2005.09.004. Epub 2005 Oct 19. Vet Microbiol. 2005. PMID: 16242273
-
In vitro effects of live and killed Brucella abortus on bovine cytokine and prostaglandin E2 production.Vet Immunol Immunopathol. 1994 Feb;40(2):149-61. doi: 10.1016/0165-2427(94)90030-2. Vet Immunol Immunopathol. 1994. PMID: 8160355
-
Expression of cytokine and Apoptosis-Associated genes in mice bone Marrow-Derived Macrophages stimulated with Brucella recombinant type IV secretion effectors.Cytokine. 2024 Oct;182:156711. doi: 10.1016/j.cyto.2024.156711. Epub 2024 Aug 1. Cytokine. 2024. PMID: 39094437
-
Immune response triggered by Brucella abortus following infection or vaccination.Vaccine. 2015 Jul 17;33(31):3659-66. doi: 10.1016/j.vaccine.2015.05.057. Epub 2015 Jun 3. Vaccine. 2015. PMID: 26048781 Review.
Cited by
-
The mechanism of chronic intracellular infection with Brucella spp.Front Cell Infect Microbiol. 2023 Apr 18;13:1129172. doi: 10.3389/fcimb.2023.1129172. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37143745 Free PMC article. Review.
-
Lipopolysaccharide mediates immuno-pathological alterations in young chicken liver through TLR4 signaling.BMC Immunol. 2017 Feb 27;18(1):12. doi: 10.1186/s12865-017-0199-7. BMC Immunol. 2017. PMID: 28241791 Free PMC article.
-
Brucella Melitensis 16M Regulates the Effect of AIR Domain on Inflammatory Factors, Autophagy, and Apoptosis in Mouse Macrophage through the ROS Signaling Pathway.PLoS One. 2016 Dec 1;11(12):e0167486. doi: 10.1371/journal.pone.0167486. eCollection 2016. PLoS One. 2016. PMID: 27907115 Free PMC article.
-
Establishment and Expression of Cytokines in a Theileria annulata-Infected Bovine B Cell Line.Genes (Basel). 2019 Apr 30;10(5):329. doi: 10.3390/genes10050329. Genes (Basel). 2019. PMID: 31052316 Free PMC article.
-
Evasion of host defense by Brucella.Cell Insight. 2023 Dec 17;3(1):100143. doi: 10.1016/j.cellin.2023.100143. eCollection 2024 Feb. Cell Insight. 2023. PMID: 38250017 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

