Response reliability observed with voltage-sensitive dye imaging of cortical layer 2/3: the probability of activation hypothesis
- PMID: 26864758
- PMCID: PMC4922466
- DOI: 10.1152/jn.00547.2015
Response reliability observed with voltage-sensitive dye imaging of cortical layer 2/3: the probability of activation hypothesis
Abstract
A central assertion in the study of neural processing is that our perception of the environment directly reflects the activity of our sensory neurons. This assertion reinforces the intuition that the strength of a sensory input directly modulates the amount of neural activity observed in response to that sensory feature: an increase in the strength of the input yields a graded increase in the amount of neural activity. However, cortical activity across a range of sensory pathways can be sparse, with individual neurons having remarkably low firing rates, often exhibiting suprathreshold activity on only a fraction of experimental trials. To compensate for this observed apparent unreliability, it is assumed that instead the local population of neurons, although not explicitly measured, does reliably represent the strength of the sensory input. This assumption, however, is largely untested. In this study, using wide-field voltage-sensitive dye (VSD) imaging of the somatosensory cortex in the anesthetized rat, we show that whisker deflection velocity, or stimulus strength, is not encoded by the magnitude of the population response at the level of cortex. Instead, modulation of whisker deflection velocity affects the likelihood of the cortical response, impacting the magnitude, rate of change, and spatial extent of the cortical response. An ideal observer analysis of the cortical response points to a probabilistic code based on repeated sampling across cortical columns and/or time, which we refer to as the probability of activation hypothesis. This hypothesis motivates a range of testable predictions for both future electrophysiological and future behavioral studies.
Keywords: VSD; coding; noise; reliability; tactile.
Copyright © 2016 the American Physiological Society.
Figures
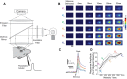

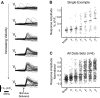


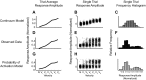


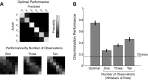

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

