Is increased hepatitis C virus case-finding combined with current or 8-week to 12-week direct-acting antiviral therapy cost-effective in UK prisons? A prevention benefit analysis
- PMID: 26864802
- PMCID: PMC4920048
- DOI: 10.1002/hep.28497
Is increased hepatitis C virus case-finding combined with current or 8-week to 12-week direct-acting antiviral therapy cost-effective in UK prisons? A prevention benefit analysis
Abstract
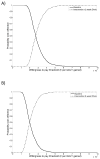
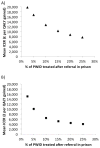
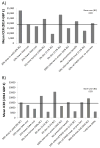
Prisoners have a high prevalence of hepatitis C virus (HCV), but case-finding may not have been cost-effective because treatment often exceeded average prison stay combined with a lack of continuity of care. We assessed the cost-effectiveness of increased HCV case-finding and treatment in UK prisons using short-course therapies. A dynamic HCV transmission model assesses the cost-effectiveness of doubling HCV case-finding (achieved through introducing opt-out HCV testing in UK pilot prisons) and increasing treatment in UK prisons compared to status quo voluntary risk-based testing (6% prison entrants/year), using currently recommended therapies (8-24 weeks) or interferon (IFN)-free direct-acting antivirals (DAAs; 8-12 weeks, 95% sustained virological response, £3300/week). Costs (British pounds, £) and health utilities (quality-adjusted life years) were used to calculate mean incremental cost-effectiveness ratios (ICERs). We assumed 56% referral and 2.5%/25% of referred people who inject drugs (PWID)/ex-PWID treated within 2 months of diagnosis in prison. PWID and ex-PWID or non-PWID are in prison an average 4 and 8 months, respectively. Doubling prison testing rates with existing treatments produces a mean ICER of £19,850/quality-adjusted life years gained compared to current testing/treatment and is 45% likely to be cost-effective under a £20,000 willingness-to-pay threshold. Switching to 8-week to 12-week IFN-free DAAs in prisons could increase cost-effectiveness (ICER £15,090/quality-adjusted life years gained). Excluding prevention benefit decreases cost-effectiveness. If >10% referred PWID are treated in prison (2.5% base case), either treatment could be highly cost-effective (ICER<£13,000). HCV case-finding and IFN-free DAAs could be highly cost-effective if DAA cost is 10% lower or with 8 weeks' duration.
Conclusions: Increased HCV testing in UK prisons (such as through opt-out testing) is borderline cost-effective compared to status quo voluntary risk-based testing under a £20,000 willingness to pay with current treatments but likely to be cost-effective if short-course IFN-free DAAs are used and could be highly cost-effective if PWID treatment rates were increased. (Hepatology 2016;63:1796-1808).
© 2016 The Authors. Hepatology published by Wiley Periodicals, Inc., on behalf of the American Association for the Study of Liver Diseases.
Conflict of interest statement
Figures
Comment in
-
Reply.Hepatology. 2016 Nov;64(5):1822-1823. doi: 10.1002/hep.28612. Epub 2016 May 28. Hepatology. 2016. PMID: 27112633 No abstract available.
-
Hepatitis C management in prisons: An insight into daily clinical practice in three major Italian correctional houses.Hepatology. 2016 Nov;64(5):1821-1822. doi: 10.1002/hep.28609. Epub 2016 May 31. Hepatology. 2016. PMID: 27118063 No abstract available.
References
-
- De Angelis D, Sweeting M, Ades AE, Hickman M, Hope V, Ramsay M. An evidence synthesis approach to estimating Hepatitis C Prevalence in England and Wales. Statistical Methods in Medical Research. 2009;18:361–379. - PubMed
-
- Dore GJ, Feld J. Hepatitis C virus therapeutic development: in pursuit of perfectovir. Clinical Infectious Diseases. 2015 in press. - PubMed
-
- Martin N, Miners A, Vickerman P. Assessing the cost-effectiveness of interventions aimed at promoting and offering hepatitis C testing in injecting drug users: an economic modelling report. 2012 http://www.nice.org.uk/nicemedia/live/11957/59552/59552.pdf.
-
- Health Protection Agency. Colindale. 2013. Hepatitis C in the UK 2013.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

