Ablation of eNOS does not promote adipose tissue inflammation
- PMID: 26864812
- PMCID: PMC4867413
- DOI: 10.1152/ajpregu.00473.2015
Ablation of eNOS does not promote adipose tissue inflammation
Abstract
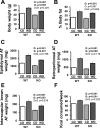
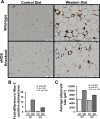
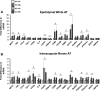
Adipose tissue (AT) inflammation is a hallmark characteristic of obesity and an important determinant of insulin resistance and cardiovascular disease; therefore, a better understanding of factors regulating AT inflammation is critical. It is well established that reduced vascular endothelial nitric oxide (NO) bioavailability promotes arterial inflammation; however, the role of NO in modulating inflammation in AT remains disputed. In the present study, 10-wk-old C57BL6 wild-type and endothelial nitric oxide synthase (eNOS) knockout male mice were randomized to either a control diet (10% kcal from fat) or a Western diet (44.9% kcal from fat, 17% sucrose, and 1% cholesterol) for 18 wk (n= 7 or 8/group). In wild-type mice, Western diet-induced obesity led to increased visceral white AT expression of inflammatory genes (e.g., MCP1, TNF-α, and CCL5 mRNAs) and markers of macrophage infiltration (e.g., CD68, ITGAM, EMR1, CD11C mRNAs, and Mac-2 protein), as well as reduced markers of mitochondrial content (e.g., OXPHOS complex I and IV protein). Unexpectedly, these effects of Western diet on visceral white AT were not accompanied by decreases in eNOS phosphorylation at Ser-1177 or increases in eNOS phosphorylation at Thr-495. Also counter to expectations, eNOS knockout mice, independent of the diet, were leaner and did not exhibit greater white or brown AT inflammation compared with wild-type mice. Collectively, these findings do not support the hypothesis that reduced NO production from eNOS contributes to obesity-related AT inflammation.
Keywords: Western diet; adipose tissue; endothelial nitric oxide synthase; obesity.
Figures

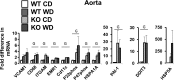
Similar articles
-
Uncoupling of Endothelial Nitric Oxide Synthase in Perivascular Adipose Tissue of Diet-Induced Obese Mice.Arterioscler Thromb Vasc Biol. 2016 Jan;36(1):78-85. doi: 10.1161/ATVBAHA.115.306263. Epub 2015 Nov 19. Arterioscler Thromb Vasc Biol. 2016. PMID: 26586660
-
Reduced vascular nitric oxide-cGMP signaling contributes to adipose tissue inflammation during high-fat feeding.Arterioscler Thromb Vasc Biol. 2011 Dec;31(12):2827-35. doi: 10.1161/ATVBAHA.111.236554. Epub 2011 Sep 8. Arterioscler Thromb Vasc Biol. 2011. PMID: 21903940 Free PMC article.
-
Overexpression of endothelial nitric oxide synthase prevents diet-induced obesity and regulates adipocyte phenotype.Circ Res. 2012 Oct 12;111(9):1176-89. doi: 10.1161/CIRCRESAHA.112.266395. Epub 2012 Aug 14. Circ Res. 2012. PMID: 22896587 Free PMC article.
-
Regulation of obesity and insulin resistance by nitric oxide.Free Radic Biol Med. 2014 Aug;73:383-99. doi: 10.1016/j.freeradbiomed.2014.05.016. Epub 2014 May 28. Free Radic Biol Med. 2014. PMID: 24878261 Free PMC article. Review.
-
eNOS, metabolic syndrome and cardiovascular disease.Trends Endocrinol Metab. 2009 Aug;20(6):295-302. doi: 10.1016/j.tem.2009.03.005. Epub 2009 Jul 31. Trends Endocrinol Metab. 2009. PMID: 19647446 Free PMC article. Review.
Cited by
-
Obesity, type 2 diabetes, and impaired insulin-stimulated blood flow: role of skeletal muscle NO synthase and endothelin-1.J Appl Physiol (1985). 2017 Jan 1;122(1):38-47. doi: 10.1152/japplphysiol.00286.2016. Epub 2016 Oct 27. J Appl Physiol (1985). 2017. PMID: 27789766 Free PMC article.
-
eNOS deletion impairs mitochondrial quality control and exacerbates Western diet-induced NASH.Am J Physiol Endocrinol Metab. 2019 Oct 1;317(4):E605-E616. doi: 10.1152/ajpendo.00096.2019. Epub 2019 Jul 30. Am J Physiol Endocrinol Metab. 2019. PMID: 31361543 Free PMC article.
-
Mechanisms of obesity-induced metabolic and vascular dysfunctions.Front Biosci (Landmark Ed). 2019 Mar 1;24(5):890-934. doi: 10.2741/4758. Front Biosci (Landmark Ed). 2019. PMID: 30844720 Free PMC article. Review.
-
A compendium of G-protein-coupled receptors and cyclic nucleotide regulation of adipose tissue metabolism and energy expenditure.Clin Sci (Lond). 2020 Mar 13;134(5):473-512. doi: 10.1042/CS20190579. Clin Sci (Lond). 2020. PMID: 32149342 Free PMC article. Review.
-
SGLT2 inhibition attenuates arterial dysfunction and decreases vascular F-actin content and expression of proteins associated with oxidative stress in aged mice.Geroscience. 2022 Jun;44(3):1657-1675. doi: 10.1007/s11357-022-00563-x. Epub 2022 Apr 15. Geroscience. 2022. PMID: 35426600 Free PMC article.
References
-
- Cayatte A, Palacino J, Horten K, Cohen R. Chronic inhibition of nitric oxide production accelerates neointima formation and impairs endothelial function in hypercholesterolemic rabbits. Arterioscler Thromb 14: 753–759, 1994. - PubMed
-
- Ginsberg HN, MacCallum PR. The obesity, metabolic syndrome, and type 2 diabetes mellitus pandemic: Part I. Increased cardiovascular disease risk and the importance of atherogenic dyslipidemia in persons with the metabolic syndrome and type 2 diabetes mellitus. J Cardiometab Syndrome 4: 113–119, 2009. - PMC - PubMed
-
- Gomez-Guzman M, Jimenez R, Sanchez M, Romero M, O'Valle F, Lopez-Sepulveda R, Quintela A, Galindo P, Zarzuelo M, Bailon E, Delpon E, Perez-Vizcaino F, Duarte J. Chronic (-)-epicatechin improves vascular oxidative and inflammatory status but not hypertension in chronic nitirc oxide-deficient rats. Br J Nutr 106: 1337–1348, 2011. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

