ATP-containing vesicles in stria vascular marginal cell cytoplasms in neonatal rat cochlea are lysosomes
- PMID: 26864824
- PMCID: PMC4750035
- DOI: 10.1038/srep20903
ATP-containing vesicles in stria vascular marginal cell cytoplasms in neonatal rat cochlea are lysosomes
Abstract
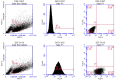
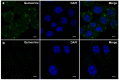
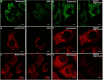
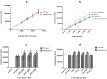
We confirmed that ATP is released from cochlear marginal cells in the stria vascular but the cell organelle in which ATP stores was not identified until now. Thus, we studied the ATP-containing cell organelles and suggest that these are lysosomes. Primary cultures of marginal cells of Sprague-Dawley rats aged 1-3 days was established. Vesicles within marginal cells stained with markers were identified under confocal laser scanning microscope and transmission electron microscope (TEM). Then ATP release from marginal cells was measured after glycyl-L-phenylalanine-ß- naphthylamide (GPN) treatment using a bioluminescent assay. Quinacrine-stained granules within marginal cells were labeled with LysoTracker, a lysosome tracer, and lysosomal-associated membrane protein 1(LAMP1), but not labeled with the mitochondrial tracer MitoTracker. Furthermore, LysoTracker-labelled puncta showed accumulation of Mant-ATP, an ATP analog. Treatment with 200 μM GPN quenched fluorescently labeled puncta after incubation with LysoTracker or quinacrine, but not MitoTracker. Quinacrine-labeled organelles observed by TEM were lysosomes, and an average 27.7 percent increase in ATP luminescence was observed in marginal cells extracellular fluid after GPN treatment. ATP-containing vesicles in cochlear marginal cells of the stria vascular from neonatal rats are likely lysosomes. ATP release from marginal cells may be via Ca(2+)-dependent lysosomal exocytosis.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




Similar articles
-
Lysosomal exocytosis of ATP is coupled to P2Y2 receptor in marginal cells in the stria vascular in neonatal rats.Cell Calcium. 2018 Dec;76:62-71. doi: 10.1016/j.ceca.2018.09.006. Epub 2018 Sep 21. Cell Calcium. 2018. PMID: 30273839
-
Release of ATP from marginal cells in the cochlea of neonatal rats can be induced by changes in extracellular and intracellular ion concentrations.PLoS One. 2012;7(10):e47124. doi: 10.1371/journal.pone.0047124. Epub 2012 Oct 10. PLoS One. 2012. PMID: 23071731 Free PMC article.
-
[Mechanism of ATP release from cultured marginal cells of stria vascularis in neonatal rat].Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2012 Jun;47(6):471-5. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2012. PMID: 22932139 Chinese.
-
Does lysosomal rupture evoke Ca2+ release? A question of pores and stores.Cell Calcium. 2020 Mar;86:102139. doi: 10.1016/j.ceca.2019.102139. Epub 2019 Dec 9. Cell Calcium. 2020. PMID: 31881482 Review.
-
Autophagy-dependent ATP release from dying cells via lysosomal exocytosis.Autophagy. 2013 Oct;9(10):1624-5. doi: 10.4161/auto.25873. Epub 2013 Aug 13. Autophagy. 2013. PMID: 23989612 Review.
Cited by
-
Quinacrine is not a vital fluorescent probe for vesicular ATP storage.Purinergic Signal. 2021 Dec;17(4):725-735. doi: 10.1007/s11302-021-09820-8. Epub 2021 Oct 28. Purinergic Signal. 2021. PMID: 34713379 Free PMC article.
-
Specific Temporal Distribution and Subcellular Localization of a Functional Vesicular Nucleotide Transporter (VNUT) in Cerebellar Granule Neurons.Front Pharmacol. 2017 Dec 22;8:951. doi: 10.3389/fphar.2017.00951. eCollection 2017. Front Pharmacol. 2017. PMID: 29311945 Free PMC article.
-
Hypoxia-adenosinergic regulation of B cell responses.Front Immunol. 2024 Nov 4;15:1478506. doi: 10.3389/fimmu.2024.1478506. eCollection 2024. Front Immunol. 2024. PMID: 39559353 Free PMC article. Review.
-
Purinergic Signalling in the Cochlea.Int J Mol Sci. 2022 Nov 28;23(23):14874. doi: 10.3390/ijms232314874. Int J Mol Sci. 2022. PMID: 36499200 Free PMC article. Review.
-
Physiopathological Role of the Vesicular Nucleotide Transporter (VNUT) in the Central Nervous System: Relevance of the Vesicular Nucleotide Release as a Potential Therapeutic Target.Front Cell Neurosci. 2019 May 17;13:224. doi: 10.3389/fncel.2019.00224. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31156398 Free PMC article. Review.
References
-
- Tritsch N. X., Yi E., Gale J. E., Glowatzki E. & Bergles D. E. The origin of spontaneous activity in the developing auditory system. Nature 450, 50–55 (2007). - PubMed
-
- Housley G. D. Extracellular nucleotide signaling in the inner ear. Mol Neurobiol. 16, 21–48 (1998). - PubMed
-
- Thorne P. R. et al. Potential role of purinergic signalling in cochlear pathology. Audiol Neurootol. 7, 180–184 (2002). - PubMed
-
- Vlajkovic S. M. et al. Noise exposure induces up-regulation of ecto-nucleoside triphosphate diphosphohydrolases 1 and 2 in rat cochlea. Neuroscience. 126, 763–773 (2004). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

