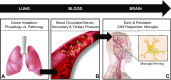
Microglial priming through the lung-brain axis: the role of air pollution-induced circulating factors
- PMID: 26864854
- PMCID: PMC4836369
- DOI: 10.1096/fj.201500047
Microglial priming through the lung-brain axis: the role of air pollution-induced circulating factors
Abstract
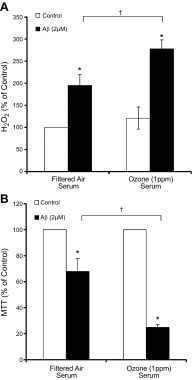
Air pollution is implicated in neurodegenerative disease risk and progression and in microglial activation, but the mechanisms are unknown. In this study, microglia remained activated 24 h after ozone (O3) exposure in rats, suggesting a persistent signal from lung to brain. Ex vivo analysis of serum from O3-treated rats revealed an augmented microglial proinflammatory response and β-amyloid 42 (Aβ42) neurotoxicity independent of traditional circulating cytokines, where macrophage-1 antigen-mediated microglia proinflammatory priming. Aged mice exhibited reduced pulmonary immune profiles and the most pronounced neuroinflammation and microglial activation in response to mixed vehicle emissions. Consistent with this premise, cluster of differentiation 36 (CD36)(-/-) mice exhibited impaired pulmonary immune responses concurrent with augmented neuroinflammation and microglial activation in response to O3 Further, aging glia were more sensitive to the proinflammatory effects of O3 serum. Together, these findings outline the lung-brain axis, where air pollutant exposures result in circulating, cytokine-independent signals present in serum that elevate the brain proinflammatory milieu, which is linked to the pulmonary response and is further augmented with age.-Mumaw, C. L., Levesque, S., McGraw, C., Robertson, S., Lucas, S., Stafflinger, J. E., Campen, M. J., Hall, P., Norenberg, J. P., Anderson, T., Lund, A. K., McDonald, J. D., Ottens, A. K., Block, M. L. Microglial priming through the lung-brain axis: the role of air pollution-induced circulating factors.
Keywords: glia; inhaled pollutants; neuroinflammation.
© FASEB.
Figures





References
-
- Block M. L., Elder A., Auten R. L., Bilbo S. D., Chen H., Chen J. C., Cory-Slechta D. A., Costa D., Diaz-Sanchez D., Dorman D. C., Gold D. R., Gray K., Jeng H. A., Kaufman J. D., Kleinman M. T., Kirshner A., Lawler C., Miller D. S., Nadadur S. S., Ritz B., Semmens E. O., Tonelli L. H., Veronesi B., Wright R. O., Wright R. J. (2012) The outdoor air pollution and brain health workshop. Neurotoxicology 33, 972–984 - PMC - PubMed
-
- Wellenius G. A., Boyle L. D., Coull B. A., Milberg W. P., Gryparis A., Schwartz J., Mittleman M. A., Lipsitz L. A. (2012) Residential proximity to nearest major roadway and cognitive function in community-dwelling seniors: results from the MOBILIZE Boston Study. J. Am. Geriatr. Soc. 60, 2075–2080 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

