Dopamine reward prediction-error signalling: a two-component response
- PMID: 26865020
- PMCID: PMC5549862
- DOI: 10.1038/nrn.2015.26
Dopamine reward prediction-error signalling: a two-component response
Abstract
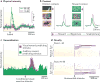
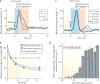
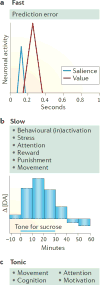
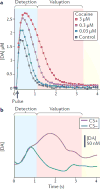
Environmental stimuli and objects, including rewards, are often processed sequentially in the brain. Recent work suggests that the phasic dopamine reward prediction-error response follows a similar sequential pattern. An initial brief, unselective and highly sensitive increase in activity unspecifically detects a wide range of environmental stimuli, then quickly evolves into the main response component, which reflects subjective reward value and utility. This temporal evolution allows the dopamine reward prediction-error signal to optimally combine speed and accuracy.
Conflict of interest statement
The author declares no competing interests.
Figures



References
-
- Schultz W. Multiple dopamine functions at different time courses. Ann. Rev. Neurosci. 2007;30:259–288. - PubMed
-
- Ljungberg T, Apicella P, Schultz W. Responses of monkey dopamine neurons during learning of behavioral reactions. J. Neurophysiol. 1992;67:145–163. - PubMed
-
- Schultz W, Dayan P, Montague RR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. - PubMed
-
- Schultz W. Predictive reward signal of dopamine neurons. J. Neurophysiol. 1998;80:1–27. - PubMed
-
- Waelti P, Dickinson A, Schultz W. Dopamine responses comply with basic assumptions of formal learning theory. Nature. 2001;412:43–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

