Modular Assembly Approach to Engineer Geometrically Precise Cardiovascular Tissue
- PMID: 26865105
- PMCID: PMC4836958
- DOI: 10.1002/adhm.201500956
Modular Assembly Approach to Engineer Geometrically Precise Cardiovascular Tissue
Abstract
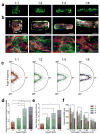
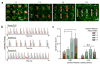
This modular assembly approach to microfabricate functional cardiovascular tissue composites enables quantitative assessment of the effects of microarchitecture on cellular function. Cardiac and endothelial modules are micromolded separately, designed to direct cardiomyocyte alignment and anisotropic contraction or vascular network formation. Assembled cardiovascular tissue composites contract synchronously, facilitating the use of this tissue-engineering platform to study structure-function relationships in the heart.
Keywords: anisotropy; cardiac tissue engineering; hydrogels; micropatterning; modular assembly.
© 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

