Dexmedetomidine post-treatment induces neuroprotection via activation of extracellular signal-regulated kinase in rats with subarachnoid haemorrhage
- PMID: 26865131
- PMCID: PMC4748945
- DOI: 10.1093/bja/aev549
Dexmedetomidine post-treatment induces neuroprotection via activation of extracellular signal-regulated kinase in rats with subarachnoid haemorrhage
Abstract
Background: Dexmedetomidine, a sedative agent, provides neuroprotection when administered during or before brain ischaemia. This study was designed to determine whether dexmedetomidine post-treatment induces neuroprotection against subarachnoid haemorrhage (SAH) and the mechanisms for this effect.
Methods: Subarachnoid haemorrhage was induced by endovascular perforation to the junction of the right middle and anterior cerebral arteries in adult rats. Dexmedetomidine was applied immediately or 2 h after onset of SAH. Neurological outcome was evaluated 2 days after SAH. Right frontal cortex area 1 was harvested 24 h after SAH for western blotting.
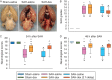
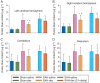
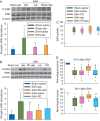
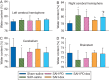
Results: Subarachnoid haemorrhage reduced neurological scores and increased brain oedema and blood-brain barrier permeability. These effects were attenuated by dexmedetomidine post-treatment. Neuroprotection by dexmedetomidine was abolished by PD98095, an inhibitor of extracellular signal-regulated kinase (ERK) activation. Phospho-ERK, the activated form of ERK, was increased by dexmedetomidine; this activation was inhibited by PD98095.
Conclusions: Dexmedetomidine post-treatment provides neuroprotection against SAH. This effect appears to be mediated by ERK.
Keywords: dexmedetomidine; extracellular signal-regulated kinase; post-treatment; subarachnoid haemorrhage.
© The Author 2016. Published by Oxford University Press on behalf of the British Journal of Anaesthesia. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures

Similar articles
-
Dexmedetomidine attenuated early brain injury in rats with subarachnoid haemorrhage by suppressing the inflammatory response: The TLR4/NF-κB pathway and the NLRP3 inflammasome may be involved in the mechanism.Brain Res. 2018 Nov 1;1698:1-10. doi: 10.1016/j.brainres.2018.05.040. Epub 2018 May 26. Brain Res. 2018. PMID: 29842860
-
Regulation of enhanced cerebrovascular expression of proinflammatory mediators in experimental subarachnoid hemorrhage via the mitogen-activated protein kinase kinase/extracellular signal-regulated kinase pathway.J Neuroinflammation. 2012 Dec 21;9:274. doi: 10.1186/1742-2094-9-274. J Neuroinflammation. 2012. PMID: 23259581 Free PMC article.
-
Aggf1 attenuates neuroinflammation and BBB disruption via PI3K/Akt/NF-κB pathway after subarachnoid hemorrhage in rats.J Neuroinflammation. 2018 Jun 9;15(1):178. doi: 10.1186/s12974-018-1211-8. J Neuroinflammation. 2018. PMID: 29885663 Free PMC article.
-
The Neuroprotection of Lysosomotropic Agents in Experimental Subarachnoid Hemorrhage Probably Involving the Apoptosis Pathway Triggering by Cathepsins via Chelating Intralysosomal Iron.Mol Neurobiol. 2015 Aug;52(1):64-77. doi: 10.1007/s12035-014-8846-y. Epub 2014 Aug 12. Mol Neurobiol. 2015. PMID: 25112680
-
Blockade of the MEK/ERK pathway with a raf inhibitor prevents activation of pro-inflammatory mediators in cerebral arteries and reduction in cerebral blood flow after subarachnoid hemorrhage in a rat model.J Cereb Blood Flow Metab. 2011 Jan;31(1):144-54. doi: 10.1038/jcbfm.2010.62. Epub 2010 Apr 28. J Cereb Blood Flow Metab. 2011. PMID: 20424636 Free PMC article.
Cited by
-
Dexmedetomidine post-conditioning protects blood-brain barrier integrity by modulating microglia/macrophage polarization via inhibiting NF-κB signaling pathway in intracerebral hemorrhage.Front Mol Neurosci. 2022 Sep 8;15:977941. doi: 10.3389/fnmol.2022.977941. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36172260 Free PMC article.
-
Dexmedetomidine Alleviates Hypoxia-Induced Synaptic Loss and Cognitive Impairment via Inhibition of Microglial NOX2 Activation in the Hippocampus of Neonatal Rats.Oxid Med Cell Longev. 2021 Feb 12;2021:6643171. doi: 10.1155/2021/6643171. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 33628369 Free PMC article.
-
Dexmedetomidine Inhibits Neuroinflammation by Altering Microglial M1/M2 Polarization Through MAPK/ERK Pathway.Neurochem Res. 2020 Feb;45(2):345-353. doi: 10.1007/s11064-019-02922-1. Epub 2019 Dec 10. Neurochem Res. 2020. PMID: 31823113
-
Inflammation and immune cell abnormalities in intracranial aneurysm subarachnoid hemorrhage (SAH): Relevant signaling pathways and therapeutic strategies.Front Immunol. 2022 Nov 23;13:1027756. doi: 10.3389/fimmu.2022.1027756. eCollection 2022. Front Immunol. 2022. PMID: 36505409 Free PMC article. Review.
-
Dexmedetomidine: an attractive adjunct to anesthesia.Korean J Anesthesiol. 2017 Aug;70(4):375-376. doi: 10.4097/kjae.2017.70.4.375. Epub 2017 Jul 13. Korean J Anesthesiol. 2017. PMID: 28794830 Free PMC article. No abstract available.
References
-
- Venti M. Subarachnoid and intraventricular hemorrhage. Front Neurol Neurosci 2012; 30: 149–53 - PubMed
-
- Hop JW, Rinkel GJ, Algra A, van Gijn J. Case-fatality rates and functional outcome after subarachnoid hemorrhage: a systematic review. Stroke 1997; 28: 660–4 - PubMed
-
- Ingall TJ, Whisnant JP, Wiebers DO, O'Fallon WM. Has there been a decline in subarachnoid hemorrhage mortality? Stroke 1989; 20: 718–24 - PubMed
-
- Kamibayashi T, Maze M. Clinical uses of α2-adrenergic agonists. Anesthesiology 2000; 93: 1345–9 - PubMed
-
- Walker SM, Howard RF, Keay KA, Fitzgerald M. Developmental age influences the effect of epidural dexmedetomidine on inflammatory hyperalgesia in rat pups. Anesthesiology 2005; 102: 1226–34 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

